Crystals of taka-amylase A, a cornerstone of protein chemistry in Japan
- PMID: 39401898
- PMCID: PMC11535007
- DOI: 10.2183/pjab.100.027
Crystals of taka-amylase A, a cornerstone of protein chemistry in Japan
Abstract
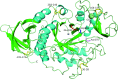
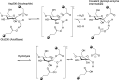
In 1935, Shiro Akabori began research on the preparation of taka-amylase A with a purity suitable for chemical research, with the intention of elucidating the chemical nature of the enzyme. He succeeded in developing a method to efficiently obtain crystallized taka-amylase A from Aspergillus oryzae. Using crystallized taka-amylase A as the starting material, a series of studies were conducted to determine its amino acid composition and sequence, sugar chain structure, and three-dimensional structure. Based on these results, the molecular structure and catalytic mechanism of taka-amylase A were elucidated. The scientific achievements from research on taka-amylase A significantly enhanced Japan's capabilities in protein research, represented by the fact that taka-amylase A was the first amylase in the world for which both chemical and crystallographic structures were elucidated.
Keywords: Shiro Akabori; enzyme; protein chemistry; protein structure; sugar chain analysis; taka-amylase A.
Figures




References
-
- Sasaki, T. (1965) Recent issues in protein research [輓近蛋白化学の研究 (綜説)]. In Opera Omnia Prof. Takaoki Sasaki [佐々木隆興先生論文集] (ed. Sasaki, T.). Sasaki Institute, Tokyo, pp. 147-170 (in Japanese).
-
- Akabori, S. (1988) People who pioneered biochemistry in Japan [日本の生化学を拓いた人々]. In What is Life? [生命 (いのち) とは--思索の断章--]. Kyoritsu Shuppan Co., Ltd., Tokyo, pp. 220-245 (in Japanese).
-
- Sumner J.B. (1926) The isolation and crystallization of the enzyme urease: Preliminary paper. J. Biol. Chem. 69, 435–441.
-
- Waldschmidt-Leitz E. (1933) The chemical nature of enzymes. Science 78, 189–190. - PubMed
-
- Sumner J.B. (1933) The chemical nature of enzymes. Science 78, 335. - PubMed

