Long-term safety profile of sutimlimab in adult Japanese patients with cold agglutinin disease
- PMID: 39402301
- PMCID: PMC11588873
- DOI: 10.1007/s12185-024-03842-9
Long-term safety profile of sutimlimab in adult Japanese patients with cold agglutinin disease
Abstract
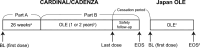
Sutimlimab, a complement inhibitor, has recently been approved in Japan for treating cold agglutinin disease (CAD). We report the safety and efficacy of sutimlimab in Japanese patients with CAD who completed a global phase 3 clinical trial (CARDINAL/CADENZA: 26-week treatment with 1-2 years of open-label extension [OLE] periods) and subsequently participated in the Japanese OLE study. Patients with a recent history of blood transfusion (CARDINAL, n = 3) and those without (CADENZA, n = 4) were analyzed (71.4% female; median [range] baseline age: 70 [46-83] years). For CARDINAL/CADENZA, the treatment duration (median [range]) was 140.9 (104.9-157.3) weeks, and the cessation period was 70 (61-133) weeks. For the Japanese OLE study, the treatment duration was 47.1 (15.1-49.1) weeks. Three (42.9%) patients experienced treatment-related and treatment-emergent adverse events (TEAEs): injection site erythema, cystitis bacterial, viral infection, and blood pressure increased during CARDINAL/CADENZA. One (14.3%) patient experienced one treatment-related TEAE (urinary tract infection) during the Japanese OLE study. One patient died of renal failure, considered unrelated to sutimlimab, that was exacerbated by hepatorenal syndrome due to liver cirrhosis and bacterial peritonitis, in addition to CKD. Hemoglobin and bilirubin levels improved during treatment but deteriorated after withdrawal and recovered on retreatment. Sutimlimab was well tolerated over a median of 3.8 years, with no new safety concerns identified during retreatment.
Keywords: Cold agglutinin disease; Complement inhibitor; Hemolytic anemia; Long-term; Sutimlimab.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: YM received research grants from Sanofi K.K., Pfizer Japan Inc., Novartis Pharma K.K., Kissei Pharmaceutical Co., Ltd., Argenx Japan K.K., Novo Nordisk Pharma Ltd., CSL Behring K.K., BioMarin Pharmaceutical Japan K.K., Chugai Pharmaceutical Co., Ltd., and Janssen Pharmaceutical K.K. and received lecture fees from Sanofi K.K., Asahi Kasei Medical Co., Ltd., Takeda Pharmaceutical Co. Ltd., Alexion Pharmaceuticals, Inc., Kissei Pharmaceutical Co. Ltd., and Kyowa Kirin Co., Ltd. and participated in advisory boards held by Sanofi K.K. and Pfizer Japan Inc and was involved in consultancy for Argenx Japan K.K., Zenyaku Holdings Co., Ltd., and Kissei Pharmaceutical Co., Ltd. YO received research grants from Mochida Pharmaceutical Co., Ltd., Fortrea Japan Inc., Janssen Pharmaceutical K.K., FUJIFILM K.K., AbbVie GK., and Otsuka Pharmaceutical Co., Ltd. and received honoraria/payment from Novartis Pharma K.K, Chugai Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., and AbbVie GK. JN received honoraria or lecture fee from Swedish Orphan Biovitrum Japan K.K, Alexion Pharmaceuticals, Inc., Roche Diagnostics K.K., Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K., BioCryst Pharmaceuticals, Inc., and Sanofi K.K. MN and OK are employees at Sanofi K.K. and ST was employee at Sanofi K.K at the time when the Japan OLE study was conducted and the data were analyzed. ES and MY have no conflict of interest to declare.
Figures


References
-
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91:460–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

