Regulation of CD8+ T cells by lipid metabolism in cancer progression
- PMID: 39402302
- PMCID: PMC11527989
- DOI: 10.1038/s41423-024-01224-z
Regulation of CD8+ T cells by lipid metabolism in cancer progression
Abstract
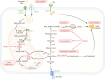
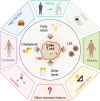
Dysregulation of lipid metabolism is a key characteristic of the tumor microenvironment, where tumor cells utilize lipids for proliferation, survival, metastasis, and evasion of immune surveillance. Lipid metabolism has become a critical regulator of CD8+ T-cell-mediated antitumor immunity, with excess lipids in the tumor microenvironment impeding CD8+ T-cell activities. Considering the limited efficacy of immunotherapy in many solid tumors, targeting lipid metabolism to enhance CD8+ T-cell effector functions could significantly improve immunotherapy outcomes. In this review, we examine recent findings on how lipid metabolic processes, including lipid uptake, synthesis, and oxidation, regulate CD8+ T cells within tumors. We also assessed the impact of different lipids on CD8+ T-cell-mediated antitumor immunity, with a particular focus on how lipid metabolism affects mitochondrial function in tumor-infiltrating CD8+ T cells. Furthermore, as cancer is a systemic disease, we examined systemic factors linking lipid metabolism to CD8+ T-cell effector function. Finally, we summarize current therapeutic approaches that target lipid metabolism to increase antitumor immunity and enhance immunotherapy. Understanding the molecular and functional interplay between lipid metabolism and CD8+ T cells offers promising therapeutic opportunities for cancer treatment.
Keywords: CD8+T cells; Immunotherapy; Lipid metabolism; Mitochondria; Oxidative phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
YK holds equity interest in KayoThera and Firebrand therapeutics.
Figures


References
-
- Swanton C, Bernard E, Abbosh C, André F, Auwerx J, Balmain A, et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell. 2024;187:1589–616. 10.1016/j.cell.2024.02.009. - PubMed
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. 10.1158/2159-8290.CD-21-1059. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

