A comprehensive analysis of the major specificities of anti-human T lymphocyte porcine immunoglobulin (p-ATG)
- PMID: 39402313
- PMCID: PMC11868322
- DOI: 10.1007/s00277-024-06028-9
A comprehensive analysis of the major specificities of anti-human T lymphocyte porcine immunoglobulin (p-ATG)
Abstract
Background: Anti-human T lymphocyte porcine immunoglobulin (p-ATG) is a potent immunosuppressive agent derived from porcine sources used in various immunotherapy applications. It is compared with similar products derived from other species, such as rabbit anti-thymocyte globulin (r-ATG) and ATG-Fresenius (ATG-F), which have distinct biological and therapeutic properties. This study aims to elucidate the mechanisms of action and comparative efficacy of p-ATG in relation to r-ATG and ATG-F through a comprehensive in vitro analysis.
Methods: A comparative analysis of p-ATG, r-ATG and ATG-F was performed, focusing on E rosette inhibitory potency, lymphocyte toxic potency, blocking activities of 24 CD molecules, and flow quantitative potency. Flow cytometric analysis was used to quantify these characteristics and assess the potency of the immunoglobulins.
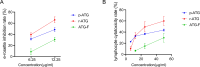
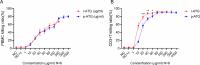
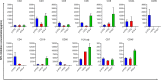
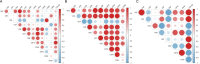
Results: p-ATG exhibited lower E rosette inhibitory and lymphocyte toxic potencies compared to r-ATG but was significantly more potent than ATG-F at equivalent concentrations. At protein concentrations above 12.5 µg/mL, p-ATG showed slightly lower potency than r-ATG and much higher potency than ATG-F in flow cytometry assays. Both p-ATG and r-ATG exhibited similar killing effects on lymphocytes within the peripheral blood mononuclear cells (PBMCs), including CD3 + T cells, with a dose-dependent response. Notably, p-ATG displayed more pronounced blocking activities against CD8, CD99, and TCR α/β compared to r-ATG.
Conclusion: p-ATG offers certain advantages over r-ATG and ATG-F, particularly in its ability to inhibit specific CD molecules and its overall potency in immunosuppressive assays, providing valuable insights for assisting clinical decision-making regarding the selection of ATG types based on patient-specific needs and treatment objectives.
Keywords: ATG-Fresenius (ATG-F); Anti-human T lymphocyte porcine immunoglobulin (p-ATG); Antibody; Horse anti-thymocyte globulin (h-ATG); Organ transplantation; Rabbit anti-thymocyte globulin (r-ATG).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The studies involving healthy human serum and PBMCs were generously provided by Tongji Medical College, Huazhong University of Science and Technology. The human samples used in this study were were reviewed and approved by the Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB2023078). Consent of publication: Not applicable. Competing interests: The authors declare that there are no commercial or financial affiliations that could be construed as a potential conflict of interest in the conduct or reporting of this research.
Figures

Similar articles
-
Recombinant anti-monkey CD3 immunotoxin depletes peripheral lymph node T lymphocytes more effectively than rabbit anti-thymocyte globulin in naïve baboons.Transpl Immunol. 2013 Dec;29(1-4):60-3. doi: 10.1016/j.trim.2013.10.004. Epub 2013 Oct 22. Transpl Immunol. 2013. PMID: 24157659 Free PMC article.
-
ATG-Fresenius inhibits blood circulating cell proliferation in a dose-dependent manner: an experimental study.Transplant Proc. 2014 Nov;46(9):3000-3. doi: 10.1016/j.transproceed.2014.07.016. Transplant Proc. 2014. PMID: 25420810
-
Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry.Transplantation. 1995 Apr 27;59(8):1194-200. Transplantation. 1995. PMID: 7732566
-
Comparison of efficacy and safety between rabbit anti-thymocyte globulin and anti-T lymphocyte globulin in kidney transplantation from donation after cardiac death: a retrospective cohort study.Nephrology (Carlton). 2015 Aug;20(8):539-43. doi: 10.1111/nep.12469. Nephrology (Carlton). 2015. PMID: 25808082
-
A review on comparing two commonly used rabbit anti-thymocyte globulins as induction therapy in solid organ transplantation.Expert Opin Biol Ther. 2013 Sep;13(9):1299-313. doi: 10.1517/14712598.2013.822064. Epub 2013 Jul 23. Expert Opin Biol Ther. 2013. PMID: 23875884 Review.
References
-
- Yang W, Liu X, Zhao X, Zhang L, Peng G, Ye L, Zhou K, Li Y, Li J, Fan H, Yang Y, Xiong Y, Jing L, Zhang F (2023) Antihuman T lymphocyte porcine immunoglobulin combined with cyclosporine as first-line immunosuppressive therapy for severe aplastic anemia in China: a large single-center, 10-year retrospective study. Ther Adv Hematol 14:20406207221146031. 10.1177/20406207221146031 - PMC - PubMed
-
- Zhu Y, Yang Y, Yang W, Song L, Li Y, Fan H, Li Y, Li J, Ye L, Zhao X, Zhou K, Peng G, Jing L, Zhang L, Zhang F (2020) Efficacy and safety of porcine ALG compared to rabbit ATG as first-line treatment for children with acquired aplastic anemia. Eur J Haematol 104(6):562–570. 10.1111/ejh.13398 - PubMed
-
- Zhang Y, Liu L, Si Y, Miao M, Qiu H, Tang X, Han Y, Fu C, Jin Z, Chen S, Sun A, Wu D (2021) A comparative study of porcine antihuman lymphocyte globulin versus antithymocyte globulin-fresenius in an allogeneic hematopoietic cell transplantation conditioning regimen for severe aplastic anemia. Hematology 26(1):741–750. 10.1080/16078454.2021.1974201 - PubMed
-
- Zhang Y, Huo J, Liu L, Shen Y, Chen J, Zhang T, Chen X, Pang A, Yang D, Zhang R, Ma Q, Zhai W, He Y, Wei J, Jiang E, Han M, Zheng Y, Feng S (2022) Comparison of hematopoietic stem cell transplantation outcomes using matched sibling donors, Haploidentical Donors, and immunosuppressive therapy for patients with acquired aplastic Anemia. Front Immunol 13:837335. 10.3389/fimmu.2022.837335 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

