Unrecognised actionability for breast cancer risk variants identified in a national-level review of Australian familial cancer centres
- PMID: 39402389
- PMCID: PMC11607406
- DOI: 10.1038/s41431-024-01705-9
Unrecognised actionability for breast cancer risk variants identified in a national-level review of Australian familial cancer centres
Abstract
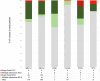
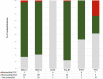
Breast cancer remains a significant global health challenge. In Australia, the adoption of publicly-funded multigene panel testing for eligible cancer patients has increased accessibility to personalised care, yet has also highlighted the increasing prevalence of variants of uncertain significance (VUS), complicating clinical decision-making. This project aimed to explore the spectrum and actionability of breast cancer VUS in Australian familial cancer centers (FCCs). Leveraging data from 11 FCCs participating in the Inherited Cancer Connect database, we retrieved VUS results from 1472 patients. Through ClinVar crosschecks and application of gene-specific ACMG/AMP guidelines, we showed the potential for reclassification of 4% of unique VUS as pathogenic or likely pathogenic, and 80% as benign or likely benign. Surveys conducted with FCCs and diagnostic laboratories described current practices and challenges in variant reclassifications, highlighting resource constraints preventing periodic VUS review and notifications from the laboratories to the FCCs. Our study suggests there are benefits to routine VUS review and reclassification, particularly in publicly-funded healthcare systems. Future research should focus on assessing the clinical impact and cost-effectiveness of implementing routine variant review practices, alongside efforts to enhance communication between FCCs and laboratories.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: This project has been approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee (HREC) under QIMR HREC Approval P1051.
Figures

References
-
- Daly MB, Pal T, Maxwell KN, Churpek J, Kohlmann W, AlHilli Z, et al. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2024. J Natl Compr Canc Netw. 2023;21:1000–10. - PubMed
-
- Blondeaux E, Arecco L, Punie K, Graffeo R, Toss A, De Angelis C, et al. Germline TP53 pathogenic variants and breast cancer: A narrative review. Cancer Treat Rev. 2023;114:102522. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

