Clinical and molecular cytogenetic findings of cat eye syndrome and a 2-year-old patient with congenital aural atresia and hearing loss
- PMID: 39402511
- PMCID: PMC11472575
- DOI: 10.1186/s12887-024-05136-9
Clinical and molecular cytogenetic findings of cat eye syndrome and a 2-year-old patient with congenital aural atresia and hearing loss
Abstract
Background: Cat eye syndrome (CES) is a rare congenital disease frequently caused by a partial tetrasomy of the proximal long (q) arm of chromosome 22, due to a small supernumerary marker chromosome (sSMC). CES patients show remarkable phenotypic variability. Despite the progress of molecular cytogenetic technology, the cause of phenotypic variability and the genotype-phenotype correlations remain unknown.
Methods: We analyzed clinical and genetic data of a new patient with CES together with 27 previously reported ones with a confirmed genomic gain in the PubMed database between 2012 and 2023.
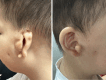
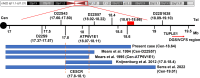
Results: We reported a boy with CES carrying a 22q11.1-q11.21 duplication of 1.76 Mb tetrasomy (16888900_18644241, hg19) who presented currently rare or unreported clinical findings such as congenital aural atresia, hearing loss, PLSVC, and IVC. The results of the whole exome sequencing (WES) showed a heterozygous mutation of the GJB2 gene (NM_004004.6: exon2: c.109G > A). In addition, the results of our literature review showed that the presence of a classical sSMC was the most frequent cytogenetic abnormality in CES (82%). 63% of cases were in a homogenous state and 37% of cases were in a mosaic state. 72% of cases had a 1-2 Mb duplication. In the majority of CES patients the breakpoints in chromosome 22 are localized to a 50 kb region (18610000_18660000 bp). The CES critical region (CESCR) may be further delimited to a 0.3 Mb region (17799398_18111588 bp). Within this region CECR2, SLC25A18, ATP6V1E1, and BCL2L13 are strong candidate genes for causing the main CES phenotype. The ear anomalies are the most frequent features in CES patients (89%) and hearing loss was present in 36% of CES patients.
Conclusions: The phenotypic features in CES are highly variable. Our findings expand the symptom spectrum of CES and lay the foundation for better delineating the clinical phenotype, molecular cytogenetic features associated with CES and genotype-phenotype correlations. We recommend performing WES to rule out the involvement of other genetic factors in the patient's phenotype. In addition, our findings also highlight the need for genetic counseling and recurrence risk assessment.
Keywords: 22q11.1q11.21 duplication; Cat eye syndrome; Congenital aural atresia; Genetic counseling; Hearing loss; Small supernumerary marker chromosome (sSMC).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Cat Eye Syndrome (Schmid-Fraccaro Syndrome).2025 Jun 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 40465813 Free Books & Documents.
-
Partial tetrasomy of chromosome 22q11.1 resulting from a supernumerary isodicentric marker chromosome in a boy with cat-eye syndrome.J Korean Med Sci. 2010 Dec;25(12):1798-801. doi: 10.3346/jkms.2010.25.12.1798. Epub 2010 Nov 24. J Korean Med Sci. 2010. PMID: 21165297 Free PMC article.
-
Cat eye syndrome: Clinical, cytogenetics and familial findings in a large cohort of 43 patients highlighting the importance of congenital heart disease and inherited cases.Am J Med Genet A. 2024 Apr;194(4):e63476. doi: 10.1002/ajmg.a.63476. Epub 2023 Nov 16. Am J Med Genet A. 2024. PMID: 37974505
-
Müllerian Agenesis in Cat Eye Syndrome and 22q11 Chromosome Abnormalities: A Case Report and Literature Review.J Pediatr Adolesc Gynecol. 2018 Apr;31(2):158-161. doi: 10.1016/j.jpag.2017.09.004. Epub 2017 Sep 14. J Pediatr Adolesc Gynecol. 2018. PMID: 28919146 Review.
-
Partial tetrasomy of chromosome 22: genetic and surgical implications for otolaryngologists.Ear Nose Throat J. 2004 Nov;83(11):748, 750, 752. Ear Nose Throat J. 2004. PMID: 15628630 Review.
References
-
- Berends MJ, Tan-Sindhunata G, Leegte B, van Essen AJ. Phenotypic variability of Cat-Eye syndrome. Genet Couns. 2001;12(1):23–34. - PubMed
-
- Liehr T, Claussen U, Starke H. Small supernumerary marker chromosomes (sSMC) in humans. Cytogenet Genome Res. 2004;107(1–2):55–67. - PubMed
-
- Glaeser AB, Diniz BL, Santos AS, Guarana BB, Muniz VF, Carlotto BS, Everling EM, Noguchi PY, Garcia AR, Miola J, et al. A child with cat-eye syndrome and oculo-auriculo-vertebral spectrum phenotype: a discussion around molecular cytogenetic findings. Eur J Med Genet. 2021;64(11):104319. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

