CD81-guided heterologous EVs present heterogeneous interactions with breast cancer cells
- PMID: 39402557
- PMCID: PMC11475557
- DOI: 10.1186/s12929-024-01084-9
CD81-guided heterologous EVs present heterogeneous interactions with breast cancer cells
Abstract
Background: Extracellular vesicles (EVs) are cell-secreted particles conceived as natural vehicles for intercellular communication. The capacity to entrap heterogeneous molecular cargoes and target specific cell populations through EV functionalization promises advancements in biomedical applications. However, the efficiency of the obtained EVs, the contribution of cell-exposed receptors to EV interactions, and the predictability of functional cargo release with potential sharing of high molecular weight recombinant mRNAs are crucial for advancing heterologous EVs in targeted therapy applications.
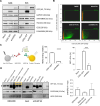
Methods: In this work, we selected the popular EV marker CD81 as a transmembrane guide for fusion proteins with a C-terminal GFP reporter encompassing or not Trastuzumab light chains targeting the HER2 receptor. We performed high-content imaging analyses to track EV-cell interactions, including isogenic breast cancer cells with manipulated HER2 expression. We validated the functional cargo delivery of recombinant EVs carrying doxorubicin upon EV-donor cell treatment. Then, we performed an in vivo study using JIMT-1 cells commonly used as HER2-refractory, trastuzumab-resistant model to detect a more than 2000 nt length recombinant mRNA in engrafted tumors.
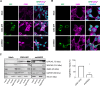
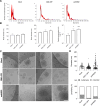
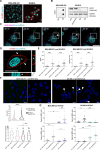
Results: Fusion proteins participated in vesicular trafficking dynamics and accumulated on secreted EVs according to their expression levels in HEK293T cells. Despite the presence of GFP, secreted EV populations retained a HER2 receptor-binding capacity and were used to track EV-cell interactions. In time-frames where the global EV distribution did not change between HER2-positive (SK-BR-3) or -negative (MDA-MB-231) breast cancer cell lines, the HER2 exposure in isogenic cells remarkably affected the tropism of heterologous EVs, demonstrating the specificity of antiHER2 EVs representing about 20% of secreted bulk vesicles. The specific interaction strongly correlated with improved cell-killing activity of doxorubicin-EVs in MDA-MB-231 ectopically expressing HER2 and reduced toxicity in SK-BR-3 with a knocked-out HER2 receptor, overcoming the effects of the free drug. Interestingly, the fusion protein-corresponding transcripts present as full-length mRNAs in recombinant EVs could reach orthotopic breast tumors in JIMT-1-xenografted mice, improving our sensitivity in detecting penetrant cargoes in tissue biopsies.
Conclusions: This study highlights the quantitative aspects underlying the creation of a platform for secreted heterologous EVs and shows the limits of single receptor-ligand interactions behind EV-cell engagement mechanisms, which now become the pivotal step to predict functional tropism and design new generations of EV-based nanovehicles.
Keywords: Drug-loading; Extracellular vesicles; Nanovehicles; RNA cargo; Receptor-binding; Tetraspanin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Gene Copy Number Dictates Extracellular Vesicle Cargo.Int J Mol Sci. 2025 Jun 8;26(12):5496. doi: 10.3390/ijms26125496. Int J Mol Sci. 2025. PMID: 40564958 Free PMC article.
-
Multiparametric profiling of HER2-enriched extracellular vesicles in breast cancer using Single Extracellular VEsicle Nanoscopy.J Nanobiotechnology. 2024 Sep 28;22(1):589. doi: 10.1186/s12951-024-02858-x. J Nanobiotechnology. 2024. PMID: 39342336 Free PMC article.
-
Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation.Mol Cancer Ther. 2018 May;17(5):1133-1142. doi: 10.1158/1535-7163.MCT-17-0827. Epub 2018 Feb 26. Mol Cancer Ther. 2018. PMID: 29483213 Free PMC article.
-
Strategies to functionalize extracellular vesicles against HER2 for anticancer activity.Extracell Vesicles Circ Nucl Acids. 2022 Apr 18;3(2):93-101. doi: 10.20517/evcna.2022.07. eCollection 2022. Extracell Vesicles Circ Nucl Acids. 2022. PMID: 39698443 Free PMC article. Review.
-
Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations.J Extracell Vesicles. 2024 Dec;13(12):e70005. doi: 10.1002/jev2.70005. J Extracell Vesicles. 2024. PMID: 39625409 Free PMC article. Review.
Cited by
-
NAMPT and NNMT released via extracellular vesicles and as soluble mediators are distinguished traits of BRAF inhibitor resistance of melanoma cells impacting on the tumor microenvironment.Cell Commun Signal. 2025 Jul 21;23(1):348. doi: 10.1186/s12964-025-02361-2. Cell Commun Signal. 2025. PMID: 40691620 Free PMC article.
-
Targeting glucocorticoid receptor signaling pathway for treatment of stress-related brain disorders.Pharmacol Rep. 2024 Dec;76(6):1333-1345. doi: 10.1007/s43440-024-00654-w. Epub 2024 Oct 3. Pharmacol Rep. 2024. PMID: 39361217 Free PMC article. Review.
References
-
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell vesicles [Internet]. 2018 [cited 2019 Feb 12];7:1535750. Available from: https://www.tandfonline.com/doi/full/10.1080/20013078.2018.1535750 - PMC - PubMed
-
- Anand S, Samuel M, Mathivanan S. Exomeres: a new member of extracellular vesicles family. Subcell Biochem. 2021;97:89–97. - PubMed
-
- Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;2:213–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

