Antimicrobial activity and nanoremediation of heavy metals using biosynthesized CS/GO/ZnO nanocomposite by Bacillus subtilis ATCC 6633 alone or immobilized in a macroporous cryogel
- PMID: 39402571
- PMCID: PMC11475717
- DOI: 10.1186/s12934-024-02535-6
Antimicrobial activity and nanoremediation of heavy metals using biosynthesized CS/GO/ZnO nanocomposite by Bacillus subtilis ATCC 6633 alone or immobilized in a macroporous cryogel
Abstract
Background: The world society is still suffering greatly from waterborne infections, with developing countries bearing most of the morbidity and death burden, especially concerning young children. Moreover, microbial resistance is one of the most prevalent global problems that extends the need for self-medication and the healing period, or it may be linked to treatment failure that results in further hospitalization, higher healthcare expenses, and higher mortality rates. Thus, innovative synthesis of new antimicrobial materials is required to preserve the environment and enhance human health.
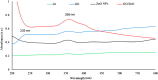
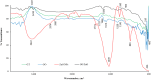
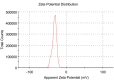
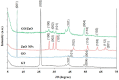
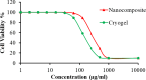
Results: The present study highlighted a simple and cost-effective approach to biosynthesize a chitosan/graphene oxide/zinc oxide nanocomposite (CS/GO/ZnO) alone and immobilized in a macroporous cryogel as a new antimicrobial agent. Bacillus subtilis ATCC 6633 was used as a safe and efficient bio-nano-factory during biosynthesis. The formation of CS/GO/ZnO was confirmed and characterized using different analyses including ultraviolet-visible spectroscopy (UV-Vis), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), selective area diffraction pattern (SADP), Zeta analyses, scanning electron microscope (SEM) and transmission electron microscopy (TEM). GO combined with ZnO NPs successfully and displayed an adsorption peak at 358 nm. The XRD results showed the crystalline composition of the loaded ZnO NPs on GO sheets. FTIR spectrum confirmed the presence of proteins during the synthesis which act as stabilizing and capping agents. The nanocomposite has a high negative surface charge (-32.8 ± 5.7 mV) which increases its stability. SEM and TEM showing the size of biosynthesized ZnO-NPs was in the range of 40-50 nm. The CS/GO/ZnO alone or immobilized in cryogel revealed good antimicrobial activities against B. cereus ATCC 14,579, Escherichia coli ATCC 25,922, and Candida albicans ATCC 10,231 in a dose-dependent manner. The CS/GO/ZnO cryogel revealed higher antimicrobial activity than GO/ZnO nanocomposite and standard antibiotics (amoxicillin and miconazole) with inhibition zones averages of 24.33 ± 0.12, 15.67 ± 0.03, and 17.5 ± 0.49 mm, respectively. The MIC values of the prepared nanocomposite against B. cereus, E. coli, and C. albicans were 80, 80, and 90 µg/ml compared to standard drugs (90, 120 and 150 µg/ml, respectively). According to the TEM ultrastructure studies of nanocomposite-treated microbes, treated cells had severe deformities and morphological alterations compared to the untreated cells including cell wall distortion, the separation between the cell wall and plasma membrane, vacuoles formation moreover complete cell lyses were also noted. In the cytotoxicity test of CS/GO/ZnO alone and its cryogel, there was a significant reduction (p˂0.05) in cell viability of WI-38 normal lung cell line after the concentration of 209 and 164 µg/ml, respectively. It showed the low toxic effect of the nanocomposite and its cryogel on the WI-38 line which implies its safety. In addition, water treatment with the CS/GO/ZnO cryogel decreased turbidity (0.58 NTU), total coliform (2 CFU/100 ml), fecal coliform (1 CFU/100 ml), fecal Streptococcus (2 CFU/100 ml), and heterotrophic plate counts (53 CFU/1 ml) not only in comparison with the chlorine-treated samples (1.69 NTU, 4 CFU/100 ml, 6 CFU/100 ml, 57 CFU/100 ml, and 140 CFU/1 ml, respectively) but also with the raw water samples (6.9 NTU, 10800 CFU/100 ml, 660 CFU/100 ml, 800 CFU/100 ml, and 4400 CFU/1 ml, respectively). Moreover, cryogel significantly decreased the concentration of different heavy metals, especially cobalt compared to chlorine (0.004 ppm, 0.002 ppm, and 0.001 ppm for raw water, chlorine-treated, and cryogel-treated groups, respectively) which helped in the reduction of their toxic effects.
Conclusion: This study provides an effective, promising, safe, and alternative nanocomposite to treat different human and animal pathogenic microbes that might be used in different environmental, industrial, and medical applications.
Keywords: Bacillus subtilis; Antimicrobial; Chitosan; Cryogel; Graphene oxide; Nanocomposite; Water treatment; Zinc oxide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Szálkai K. Water-borne diseases. In: Romaniuk S, Thapa M, Marton P, editors. The Palgrave encyclopedia of global security studies. Cham, Switzerland: Springer International Publishing; 2019. pp. 1540–6. 10.1007/978-3-319-74319-6.
-
- Edokpayi JN, Odiyo JO, Durowoju OS. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. In: Water quality. Vienna, Austria: IntechOpen; 2017. pp. 401–416. 10.5772/66561
-
- WHO (World Health Organization). Drinking-water. https://www.who.int/news-room/fact-sheets/detail/drinking-water. Accessed 15 Nov 2021.
-
- Prüss-Üstün A, Bos R, Gore F, editors. Safer water, better health: costs, benefits and sustainability of interventions to protect and promote health. Geneva, Switzerland: WHO; 2008. http://whqlibdoc.who.int/publications/2008/9789241596435_eng.pdf
-
- Huertas E, Salgot M, Hollender J, Weber S, Dott W, Khan S, Schaefer A, Messalem R, Bis B, Aharoni A, Chikurel H. Key objectives for water reuse concepts. Desalination. 2008;218:120–31. 10.1016/j.desal.2006.09.032. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

