Mixed active metabolites of the SNP-6 series of novel compounds mitigate metabolic dysfunction-associated steatohepatitis and fibrosis: promising results from pre-clinical and clinical trials
- PMID: 39402603
- PMCID: PMC11476197
- DOI: 10.1186/s12967-024-05686-7
Mixed active metabolites of the SNP-6 series of novel compounds mitigate metabolic dysfunction-associated steatohepatitis and fibrosis: promising results from pre-clinical and clinical trials
Abstract
Background: Metabolic dysfunction-associated steatohepatitis (MASH) is a growing global health concern with no effective pharmacological treatments. SNP-630, a newly developed synthetic molecule with multiple mechanisms of action, and a mixture of two of its active metabolites (SNP-630-MS) inhibit CYP2E1 expression to prevent reactive oxygen species generation, thereby reducing the accumulation of hepatic triglycerides and lowering chemokine levels. This study investigated the SNP-630's potential to alleviate the liver injury in MASH and its efficacy in both a mouse model and patients with MASH to identify a drug candidate that targets multiple pathways implicated in MASH.
Methods: SNP-630 and SNP-630-MS were separately administered to the MASH mouse model. The tolerability, safety, and efficacy of SNP-630-MS were also evaluated in 35 patients with MASH. The primary endpoint of the study was assessment of the changes in serum alanine aminotransferase (ALT) levels from baseline to week 12, while the secondary endpoints included the evaluation of liver inflammation, steatosis, and fibrosis parameters and markers.
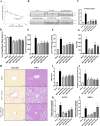
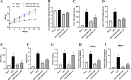
Results: SNP-630 treatment in mice improved inflammation, liver steatosis, and fibrosis compared with that in the MASH control group. Both SNP-630 and SNP-630-MS treatments markedly reduced ALT levels, hepatic triglyceride content, and the expression of inflammatory cytokines monocyte chemoattractant protein 1 and fibrotic collagen (i.e., Col1a1, Col3a1, and Timp1) in mice. In the clinical trial, patients treated with SNP-630-MS exhibited significant improvement in ALT levels at week 12 compared with baseline levels, with no reports of severe adverse events. This improvement in ALT levels surpassed that achieved with most other MASH candidates. SNP-630-MS demonstrated potential antifibrotic effects, as evidenced by a significant decrease in the levels of fibrogenesis-related biomarkers such as CCL4, CCL5, and caspase 3. Subgroup analysis using FibroScan measurements further indicated the efficacy of SNP-630-MS in ameliorating liver fibrosis.
Conclusions: SNP-630 and SNP-630-MS demonstrated favorable results in mice. SNP-630-MS showed excellent tolerability in mice and patients with MASH. Efficacy analyses indicated that SNP-630-MS improved liver steatosis and injury in patients with MASH, suggesting that SNP-630 and 630-MS are promising therapeutic options for MASH. Larger scale clinical trials remain warranted to assess the efficacy and safety of SNP-630 in MASH.
Trial registration: ClinicalTrials.gov NCT03868566. Registered 06 March 2019-Retrospectively registered, https://clinicaltrials.gov/study/NCT03868566.
Keywords: Alanine aminotransferase; Metabolic dysfunction-associated steatohepatitis; SNP-630.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Sanjay KV, Vishwakarma S, Zope BR, Mane VS, Mohire S, Dhakshinamoorthy S. ATP citrate lyase inhibitor Bempedoic Acid alleviate long term HFD induced NASH through improvement in glycemic control, reduction of hepatic triglycerides & total cholesterol, modulation of inflammatory & fibrotic genes and improvement in NAS score. Curr Res Pharmacol Drug Discov. 2021;2: 100051. 10.1016/j.crphar.2021.100051. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

