Comparison of 75 mg versus 150 mg aspirin for the prevention of preterm preeclampsia in high-risk women at a tertiary level hospital: study protocol for a randomized double-blind clinical trial
- PMID: 39402669
- PMCID: PMC11476763
- DOI: 10.1186/s13063-024-08520-z
Comparison of 75 mg versus 150 mg aspirin for the prevention of preterm preeclampsia in high-risk women at a tertiary level hospital: study protocol for a randomized double-blind clinical trial
Abstract
Background: Hypertensive disorders of pregnancy (HDP) pose significant risks to maternal and fetal health, with substantial mortality and morbidity rates globally, particularly in developing countries. Pre-eclampsia (PE) accounts for a notable portion of maternal morbidity and mortality, with varied prevalence across regions within countries like India. Despite advancements, disparities in healthcare access persist, influencing outcomes. PE not only affects maternal health during pregnancy but also predisposes women to long-term cardiovascular complications, emphasizing the need for early screening and preventive measures.
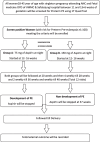
Methods: This prospective randomized double-blind clinical trial aims to compare the efficacy and safety of 75 mg versus 150 mg aspirin for preventing preterm pre-eclampsia in high-risk women. Screen-positive women aged 18-45 years with singleton pregnancies between 12 and 16 weeks of gestational age will be enrolled. They will be randomized in a 1:1 ratio to receive either 75 mg or 150 mg of aspirin nightly until 37 weeks of pregnancy or earlier if preterm pre-eclampsia develops. Feto-maternal outcomes, including preterm pre-eclampsia incidence and neonatal and maternal complications, will be assessed. The sample size calculation based on expected proportions of preterm pre-eclampsia in both groups indicates a total of 370 participants (185 per group) accounting for 20% attrition.
Discussion: This prospective randomized double-blind clinical trial aims to compare the effectiveness and safety of two doses of aspirin (75 mg vs 150 mg) in preventing preterm pre-eclampsia in high-risk women. The potential implications of this study are significant, including the optimization of aspirin prophylaxis, the development of evidence-based guidelines, and comprehensive assessment of maternal and fetal outcomes. In conclusion, the results of this study have the potential to significantly impact clinical practice by enhancing maternal and perinatal health outcomes and contributing to evidence-based obstetric care.
Trial registration: Clinical Trials Registry-India CTRI/2023/12/060983. Trial was registered prospectively on 29 December 2023. Acknowledgement Number REF/2023/12/076358. https://acrobat.adobe.com/id/urn:aaid:sc:AP:15870322-f1f4-4460-900c-6e056ab83a44 .
Keywords: Hypertensive disorders of pregnancy; Aspirin prophylaxis; Clinical trial; Evidence-Based practice; Fetal outcomes; High-Risk women; Maternal outcomes; Obstetric care; Pre-Eclampsia; Preterm pre-Eclampsia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Van Doorn R, Mukhtarova N, Flyke IP, Lasarev M, Kim K, Hennekens CH, et al. Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: a systematic review and meta-analysis. PLoS ONE. 2021;16(3):e0247782. 10.1371/journal.pone.0247782.PMID:33690642;PMCID:PMC7943022. - DOI - PMC - PubMed
-
- Sisti G, Fochesato C, Elkafrawi D, Marcus B, Schiattarella A. Is blood pressure 120–139/80-89 mmHg before 20 weeks a risk factor for hypertensive disorders of pregnancy? A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2023;284:66–75. 10.1016/j.ejogrb.2023.03.011. Epub 2023 Mar 15 PMID: 36934679. - DOI - PubMed
-
- Dhinwa M, Gawande K, Jha N, Anjali M, Bhadoria AS, Sinha S. Prevalence of hypertensive disorders of pregnancy in India: a systematic review and meta-analysis. Journal of Medical Evidence. 2021;2(2):105. 10.4103/JME.JME_168_20. - DOI
-
- Magee LA, Sharma S, Nathan HL, Adetoro OO, Bellad MB, Goudar S, et al; CLIP Study Group. The incidence of pregnancy hypertension in India, Pakistan, Mozambique, and Nigeria: a prospective population-level analysis. PLoS Med. 2019 Apr 12;16(4):e1002783. 10.1371/journal.pmed.1002783. PMID: 30978179; PMCID: PMC6461222. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources