iPSC-derived megakaryocytes and platelets accelerate wound healing and angiogenesis
- PMID: 39402677
- PMCID: PMC11477011
- DOI: 10.1186/s13287-024-03966-z
iPSC-derived megakaryocytes and platelets accelerate wound healing and angiogenesis
Abstract
Background: Platelet-rich plasma (PRP), which is prepared by concentrating platelets in autologous blood, shows efficacy in chronic skin wounds via multiple growth factors. However, it exhibits heterogeneity across patients, leading to unstable therapeutic efficacy. Human induced pluripotent stem cell (iPSC)-derived megakaryocytes and platelets (iMPs) are capable of providing a stable supply, holding promise as materials for novel platelet concentrate-based therapies. In this context, we evaluated the effect of iMPs on wound healing and validated lyophilization for clinical applications.
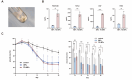
Methods: The growth factors released by activated iMPs were measured. The effect of the administration of iMPs on human fibroblasts and human umbilical vein endothelial cells (HUVECs) was investigated in vitro. iMPs were applied to dorsal skin defects of diabetic mice to assess the wound closure rate and quantify collagen deposition and angiogenesis. Following the storage of freeze-dried iMPs (FD-iMPs) for three months, the stability of growth factors and their efficacy in animal models were determined.
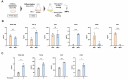
Result: Multiple growth factors that promote wound healing were detected in activated iMPs. iMPs specifically released FGF2 and exhibited a superior enhancement of HUVEC proliferation compared to PRP. Moreover, an RNA-seq analysis revealed that iMPs induce polarization to stalk cells and enhance ANGPTL4 gene expression in HUVECs. Animal studies demonstrated that iMPs promoted wound closure and angiogenesis in chronic wounds caused by diabetes. We also confirmed the long-term stability of growth factors in FD-iMPs and their comparable effects to those of original iMPs in the animal model.
Conclusion: Our study demonstrates that iMPs promote angiogenesis and wound healing through the activation of vascular endothelial cells. iMPs exhibited more effectiveness than PRP, an effect attributed to the exclusive presence of specific factors including FGF2. Lyophilization enabled the long-term maintenance of the composition of the growth factors and efficacy of the iMPs, therefore contributing to stable supply for clinical application. These findings suggest that iMPs provide a novel treatment for chronic wounds.
Keywords: Growth factors; Immortalized megakaryocyte cell lines; Lyophilization; Platelets; Wound healing.
© 2024. The Author(s).
Conflict of interest statement
K.K., N.T., M.M. and K.E. hold stock of Kineplat Co.Ltd. that has a related patent of this research.
Figures





References
-
- Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7(5):335–46. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

