Enzyme-Mediated Organic Neurohybrid Synapses
- PMID: 39402777
- PMCID: PMC11619221
- DOI: 10.1002/adma.202409614
Enzyme-Mediated Organic Neurohybrid Synapses
Abstract
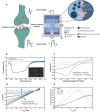
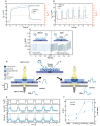
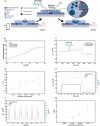
The development of organic artificial synapses that exhibit biomimicry features also may enable a more seamless integration of neuroelectronic devices in the nervous system, allowing artificial neuromodulation to be perceived as natural behavior by neuronal cells. Nevertheless, the capability to interact with both electroactive and non-electroactive neurotransmitters remains a challenge since state-of-the-art devices mainly rely on the oxidation of electroactive species. Here, the study proposes an organic artificial synapse engineered to enable interaction with non-electroactive species present in the central nervous system. By integrating a conductive polymeric film functionalized with platinum nanoparticles, the device can catalyze the oxidation of electroactive molecules (i.e., H2O2) resulting from neurotransmitter-specific enzymatic reactions following an enzymatic functionalization, therefore exhibiting neuromorphic functions driven by non-electroactive neurotransmitters. The creation of devices that can interact with or monitor these neurotransmitters can be seen as a significant step toward innovative technologies to expand the understanding of the mechanisms underlying neurological disorders and the development of novel, more effective treatments for them.
Keywords: electrochemical neuromorphic organic devices; glutamate sensing; neuroelectronics.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gkoupidenis P., Zhang Y., Kleemann H., Ling H., Santoro F., Fabiano S., Salleo A., Van De Burgt Y., Nat Rev Mater 2023, 9, 134.
-
- Lee Y., Lee T.‐W., Acc. Chem. Res. 2019, 52, 964. - PubMed
-
- Jastrzebska‐Perfect P., Chowdhury S., Spyropoulos G. D., Zhao Z., Cea C., Gelinas J. N., Khodagholy D., Adv. Funct. Mater. 2020, 30, 1909165.
-
- Go G.‐T., Lee Y., Seo D.‐G., Lee T.‐W., Adv. Mater. 2022, 34, 2201864. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

