Ceramide lowering rescues respiratory defects in a Drosophila model of acid sphingomyelinase deficiency
- PMID: 39402882
- PMCID: PMC11630749
- DOI: 10.1093/hmg/ddae143
Ceramide lowering rescues respiratory defects in a Drosophila model of acid sphingomyelinase deficiency
Abstract
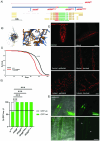
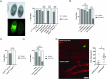
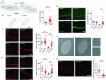
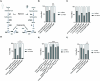
Types A and B Niemann-Pick disease (NPD) are inherited multisystem lysosomal storage disorders due to mutations in the SMPD1 gene. Respiratory dysfunction is a key hallmark of NPD, yet the mechanism for this is underexplored. SMPD1 encodes acid sphingomyelinase (ASM), which hydrolyses sphingomyelin to ceramide and phosphocholine. Here, we present a Drosophila model of ASM loss-of-function, lacking the fly orthologue of SMPD1, dASM, modelling several aspects of the respiratory pathology of NPD. dASM is expressed in the late-embryonic fly respiratory network, the trachea, and is secreted into the tracheal lumen. Loss of dASM results in embryonic lethality, and the tracheal lumen fails to fill normally with gas prior to eclosion. We demonstrate that the endocytic clearance of luminal constituents prior to gas-filling is defective in dASM mutants, and is coincident with autophagic, but not lysosomal defects, in late stage embryonic trachea. Finally, we show that although bulk sphingolipids are unchanged, dietary loss of lipids in combination with genetic and pharmacological block of ceramide synthesis rescues the airway gas-filling defects. We highlight myriocin as a potential therapeutic drug for the treatment of the developmental respiratory defects associated with ASM deficiency, and present a new NPD model amenable to genetic and pharmacological screens.
Keywords: Drosophila; SMPD1; dASM; Niemann-Pick; lysosomal storage disorder; lysosome; sphingolipid.
© The Author(s) 2024. Published by Oxford University Press.
Figures

References
-
- Zampieri S, Filocamo M, Pianta A. et al. SMPD1 mutation update: database and comprehensive analysis of published and novel variants. Hum Mutat 2016;37:139–147. - PubMed
-
- Stenson PD, Ball EV, Mort M. et al. Human gene mutation database (HGMD): 2003 update. Hum Mutat 2003;21:577–581. - PubMed
-
- Gan-Or Z, Orr-Urtreger A, Alcalay RN. et al. The emerging role of SMPD1 mutations in Parkinson's disease: implications for future studies. Parkinsonism Relat Disord 2015;21:1294–1295. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

