Non-Surgical Treatment of Moderate Periodontal Intrabony Defects With Adjunctive Cross-Linked Hyaluronic Acid: A Single-Blinded Randomized Controlled Clinical Trial
- PMID: 39402910
- PMCID: PMC11743238
- DOI: 10.1111/jcpe.14078
Non-Surgical Treatment of Moderate Periodontal Intrabony Defects With Adjunctive Cross-Linked Hyaluronic Acid: A Single-Blinded Randomized Controlled Clinical Trial
Abstract
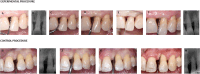
Aim: To evaluate the clinical outcomes of moderate intrabony defects treated with minimally invasive non-surgical technique (MINST) with or without adjunctive delivery of cross-linked hyaluronic acid (xHyA) gel.
Materials and methods: Forty-two patients with 42 interdental intrabony defects were randomly assigned to test (MINST + xHyA) or control procedures (MINST alone). Probing depth (PD), clinical attachment level (CAL), gingival recession (GR) and bleeding on probing (BOP) at the treated sites were assessed at baseline and at 3 and 6 months. Full-mouth plaque score (FMPS) and full-mouth bleeding score (FMBS) were recorded at baseline and after 6 months. Radiographic evaluation was performed at baseline and after 6 months, assessing the defect fill (DF) and radiographic defect angle (RDA). The primary outcome variable was PD change.
Results: Thirty-eight patients completed the trial without any adverse events. At 6 months, a statistically significant improvement (p < 0.05) was measured in all clinical parameters except GR (p > 0.05). However, no statistically significant differences were found between the experimental and control procedures (p > 0.05). Statistically significant differences between the test and control sites were observed at 3 months for PD and CAL changes (p < 0.05). The DF change was statistically significant when comparing experimental and control procedures at 6 months (p < 0.05). Both procedures failed to show statistically significant differences in terms of RDA changes at 6 months (p > 0.05).
Conclusion: Within their limitations, the present results indicate that (a) treatment of intrabony defects with MINST, with or without application of xHyA gel, resulted in statistically significant improvements in the investigated clinical parameters at 3 and 6 months after therapy, and (b) although the adjunctive use of xHyA gel to MINST improved the clinical outcomes compared with MINST alone up to 3 months, statistically significant differences were not observed at 6 months. The study protocol was registered in ClinicalTrial.gov (NCT05188898).
Keywords: hyaluronic acid; intrabony defects; minimally invasive; periodontitis; subgingival instrumentation.
© 2024 The Author(s). Journal of Clinical Periodontology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Barbato, L. , Selvaggi F., Kalemaj Z., et al. 2020. “Clinical Efficacy of Minimally Invasive Surgery (MIS) and Non‐Surgical (MINST) Treatments of Periodontal Intrabony Defects. A Systematic Review and Network Meta‐Analysis of RCT's.” Clinical Oral Investigations 24: 1125–1135. 10.1007/s00784-020-03229-0. - DOI - PubMed
-
- Bezerra, D. B. , Brazao M. A. M., de Campos M. L. G., Casati M. Z., Sallum E. A., and Sallum A. W.. 2012. “Association of Hyaluronic Acid With a Collagen Scaffold May Improve Bone Healing in Critical‐Size Bone Defects.” Clinical Oral Implants Research 23: 938–942. 10.1111/j.0501.2011.02234.x. - DOI - PubMed
-
- Claffey, N. , Nylund K., Kiger R., Garrett S., and Egelberg J.. 1990. “Diagnostic Predictability of Scores of Plaque, Bleeding, Suppuration and Probing Depth for Probing Attachment Loss. 3½ Years of Observation Following Initial Periodontal Therapy.” Journal of Clinical Periodontology 17: 108–114. 10.1111/j.1600-051x.1990.tb01071.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

