Is the GFR-based scaling approach adequate for predicting pediatric renal clearance of drugs with passive tubular reabsorption? Insights from PBPK modeling
- PMID: 39403008
- PMCID: PMC11706418
- DOI: 10.1002/psp4.13254
Is the GFR-based scaling approach adequate for predicting pediatric renal clearance of drugs with passive tubular reabsorption? Insights from PBPK modeling
Abstract
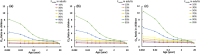
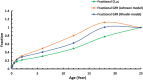
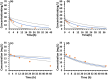
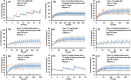
Empirical maturation models (e.g., Johnson and Rhodin models) for glomerular filtration rate (GFR) are commonly used as scaling factors for predicting pediatric renal clearance, but their predictive performance for drugs featured with tubular reabsorption is poorly understood. This study investigated the adequacy of GFR-based scaling models for predicting pediatric renal clearance in drugs with passive tubular reabsorption by comparing with a mechanistic kidney model (Mech-KiM) that encompasses the physiological processes of glomerular filtration, tubular secretion, and reabsorption. The analysis utilized hypothetical drugs with varying fractions of tubular reabsorption (Freabs), alongside the model drug metronidazole, which has a Freabs of 96%. Our simulations showed that when Freabs is ≤70%, the discrepancies between the GFR-based scaling methods and the Mech-KiM model in predicting pediatric renal clearance were generally within a twofold range throughout childhood. However, for drugs with substantial tubular reabsorption (e.g., Freabs > 70%), discrepancies greater than twofold were observed between the GFR-based scaling methods and the Mech-KiM model in predicting renal clearance for young children. In neonates, the differences ranged from 5- to 10-fold when the adult Freabs was 95%. Pediatric physiologically based pharmacokinetic (PBPK) modeling of metronidazole revealed that using a GFR-based scaling method (Johnson model) significantly overestimated drug concentrations in children under 2 months, whereas utilizing the Mech-KiM model for renal clearance predictions yielded estimates closely aligned with observed concentrations. Our study demonstrates that using GFR-based scaling models to predict pediatric renal clearance might be inadequate for drugs with extensive passive tubular reabsorption (e.g., Freabs > 70%).
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors declared no competing interests for this work.
Figures
Similar articles
-
Novel minimal physiologically-based model for the prediction of passive tubular reabsorption and renal excretion clearance.Eur J Pharm Sci. 2016 Oct 30;94:59-71. doi: 10.1016/j.ejps.2016.03.018. Epub 2016 Mar 28. Eur J Pharm Sci. 2016. PMID: 27033147 Free PMC article.
-
Novel Mechanistic PBPK Model to Predict Renal Clearance in Varying Stages of CKD by Incorporating Tubular Adaptation and Dynamic Passive Reabsorption.CPT Pharmacometrics Syst Pharmacol. 2020 Oct;9(10):571-583. doi: 10.1002/psp4.12553. Epub 2020 Sep 25. CPT Pharmacometrics Syst Pharmacol. 2020. PMID: 32977369 Free PMC article.
-
The Predictive Value of Glomerular Filtration Rate-Based Scaling of Pediatric Clearance and Doses for Drugs Eliminated by Glomerular Filtration with Varying Protein-Binding Properties.Clin Pharmacokinet. 2020 Oct;59(10):1291-1301. doi: 10.1007/s40262-020-00890-2. Clin Pharmacokinet. 2020. PMID: 32314184 Free PMC article.
-
A review of physiologically based pharmacokinetic modeling of renal drug disposition.Drug Metab Dispos. 2025 Mar;53(3):100042. doi: 10.1016/j.dmd.2025.100042. Epub 2025 Jan 21. Drug Metab Dispos. 2025. PMID: 40048993 Review.
-
Renal clearance parameters for PBPK model analysis of early lifestage differences in the disposition of environmental toxicants.Regul Toxicol Pharmacol. 2008 Jun;51(1):66-86. doi: 10.1016/j.yrtph.2008.02.005. Epub 2008 Feb 29. Regul Toxicol Pharmacol. 2008. PMID: 18433959 Review.
References
-
- Chase SL, Sutton JD. Lisinopril: a new angiotensin‐converting enzyme inhibitor. Pharmacotherapy. 1989;9:120‐128; discussion 128–130. - PubMed
-
- Li S, Xie F. Foetal and neonatal exposure prediction and dosing evaluation for ampicillin using a physiologically‐based pharmacokinetic modelling approach. Br J Clin Pharmacol. 2023;89:1402‐1412. - PubMed
-
- Shen DD, Azarnoff DL. Clinical pharmacokinetics of methotrexate. Clin Pharmacokinet. 1978;3:1‐13. - PubMed
-
- Talevi A, Bellera CL. Renal drug excretion. The ADME Encyclopedia: A Comprehensive Guide on Biopharmacy and Pharmacokinetics. Springer International Publishing; 2021.
-
- Fagerholm U. Prediction of human pharmacokinetics – renal metabolic and excretion clearance. J Pharm Pharmacol. 2007;59:1463‐1471. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

