Clinical Trial Design Considerations for Hospitalised Patients With Ulcerative Colitis Flares and Application to Study Hyperbaric Oxygen Therapy in the NIDDK HBOT-UC Consortium
- PMID: 39403018
- PMCID: PMC11599782
- DOI: 10.1111/apt.18326
Clinical Trial Design Considerations for Hospitalised Patients With Ulcerative Colitis Flares and Application to Study Hyperbaric Oxygen Therapy in the NIDDK HBOT-UC Consortium
Abstract
Background: Patients with ulcerative colitis (UC) who are hospitalised for acute severe flares represent a high-risk orphan population.
Aim: To provide guidance for clinical trial design methodology in these patients.
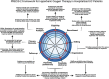
Methods: We created a multi-centre consortium to design and conduct a clinical trial for a novel therapeutic intervention (hyperbaric oxygen therapy) in patients with UC hospitalised for moderate-severe flares. During planning, we identified and addressed specific gaps for inclusion/exclusion criteria; disease activity measures; pragmatic trial design considerations within care pathways for hospitalised patients; standardisation of care delivery; primary and secondary outcomes; and sample size and statistical analysis approaches.
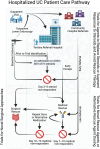
Results: The Truelove-Witt criteria should not be used in isolation. Endoscopy is critical for defining eligible populations. Patient-reported outcomes should include rectal bleeding and stool frequency, with secondary measurement of urgency and nocturnal bowel movements. Trial design needs to be tailored to care pathways, with early intervention focused on replacing and/or optimising responsiveness to steroids and later interventions focused on testing novel rescue agents or strategies. The PRECIS-2 framework offers a means of tailoring to local populations. We provide standardisation of baseline testing, venous thromboprophylaxis, steroid dosing, discharge criteria and post-discharge follow-up to avoid confounding by usual care variability. Statistical considerations are provided given the small clinical trial nature of this population.
Conclusion: We provide an outline for framework decisions made for the hyperbaric oxygen trial in patients hospitalised for UC flares. Future research should focus on the remaining gaps identified.
© 2024 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
References
-
- Fumery M., Singh S., Dulai P. S., Gower‐Rousseau C., Peyrin‐Biroulet L., and Sandborn W. J., “Natural History of Adult Ulcerative Colitis in Population‐Based Cohorts: A Systematic Review,” Clinical Gastroenterology and Hepatology 16, no. 3 (2018): 343–356.e3, 10.1016/j.cgh.2017.06.016. - DOI - PMC - PubMed
-
- Ma C., Smith M. K., Guizzetti L., et al., “Assessing National Trends and Disparities in Ambulatory, Emergency Department, and Inpatient Visits for Inflammatory Bowel Disease in the United States (2005‐2016),” Clinical Gastroenterology and Hepatology 18, no. 11 (2020): 2500–2509.e1, 10.1016/j.cgh.2020.01.023. - DOI - PMC - PubMed
-
- Nguyen N. H., Koola J., Dulai P. S., Prokop L. J., Sandborn W. J., and Singh S., “Rate of Risk Factors for and Interventions to Reduce Hospital Readmission in Patients With Inflammatory Bowel Diseases,” Clinical Gastroenterology and Hepatology 18 (2019): 1939–1948.e7, 10.1016/j.cgh.2019.08.042. - DOI - PMC - PubMed
