Ramucirumab-induced ascites with endothelial growth factor receptor mutation-positive non-small cell lung cancer: Two case reports
- PMID: 39403090
- PMCID: PMC11473188
- DOI: 10.1002/rcr2.70046
Ramucirumab-induced ascites with endothelial growth factor receptor mutation-positive non-small cell lung cancer: Two case reports
Abstract
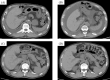
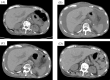
Ramucirumab (RAM) has been approved for the treatment of non-small cell lung cancer (NSCLC). Here, we report two cases of RAM-induced ascites with epidermal growth factor receptor-mutant NSCLC. Patient 1, a 72-year-old man, developed ascites 20 months after erlotinib (ERL) and RAM administration, which resolved after their discontinuation and performing paracentesis. Patient 2, an 83-year-old woman, developed ascites 9 months after ERL and RAM administration, which resolved after RAM discontinuation and furosemide administration. Ramucirumab administration can cause ascites due to increased hepatic sinusoidal pressure. Clinicians should be aware of RAM-induced ascites in patients with NSCLC and should appropriately manage it.
Keywords: ascites; epidermal growth factor receptor; erlotinib; non‐small cell lung cancer; ramucirumab.
© 2024 The Author(s). Respirology Case Reports published by John Wiley & Sons Australia, Ltd on behalf of The Asian Pacific Society of Respirology.
Conflict of interest statement
None declared.
Figures
References
-
- Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second‐line treatment in patients with advanced hepatocellular carcinoma following first‐line therapy with sorafenib (REACH): a randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. - PubMed
-
- Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz‐Ares L, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR‐mutated, advanced non‐small‐cell lung cancer (RELAY): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. - PubMed
-
- Yoshiji H, Kuriyama S, Hicklin DJ, Huber J, Yoshii J, Ikenaka Y, et al. The vascular endothelial growth factor receptor KDR/Flk‐1 is a major regulator of malignant ascites formation in the mouse hepatocellular carcinoma model. Hepatology. 2001;33:841–847. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

