Photoswitchable TRPC6 channel activators evoke distinct channel kinetics reflecting different gating behaviors
- PMID: 39403196
- PMCID: PMC11472632
- DOI: 10.1016/j.isci.2024.111008
Photoswitchable TRPC6 channel activators evoke distinct channel kinetics reflecting different gating behaviors
Abstract
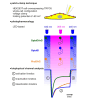
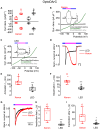
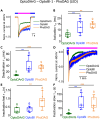
The non-selective transient receptor potential canonical 6 (TRPC6) cation channels have several physiological and pathophysiological effects. They are activated by the lipid second messenger diacylglycerol (DAG) and by non-lipidic compounds such as GSK 1702934A (GSK). Advances in photopharmacology led to the development of photoswitchable activators such as PhoDAG, OptoDArG, and OptoBI-1 that can be switched ON and OFF with the spatiotemporal precision of light. We aimed to elucidate whether these photopharmaceuticals allow for a reliable determination of the ion channel current kinetics. We performed electrophysiological whole-cell measurements in the overexpression system and analyzed TRPC6 currents induced by photoswitching. We observed distinct activation, deactivation and inactivation current kinetics suggesting that each photoswitchable activator elicits a distinct active channel state. Notably, the current kinetics strongly depended on the intensity of the light source. Altogether, photopharmaceuticals are advantageous for an extended biophysical characterization of whole-cell currents and provide insight into their gating mechanism.
Keywords: Biological sciences; Biophysical chemistry; Biophysics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Storch U., Forst A.L., Pardatscher F., Erdogmus S., Philipp M., Gregoritza M., Mederos y Schnitzler M., Gudermann T. Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc. Natl. Acad. Sci. USA. 2017;114:E37–E46. doi: 10.1073/pnas.1612263114. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

