Anion-dependent ion-pairing assemblies of triazatriangulenium cation that interferes with stacking structures
- PMID: 39403304
- PMCID: PMC11472656
- DOI: 10.3762/bjoc.20.215
Anion-dependent ion-pairing assemblies of triazatriangulenium cation that interferes with stacking structures
Abstract
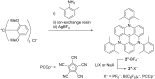
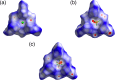
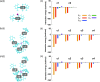
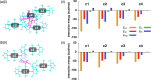
Ion pairs of N-(2,6-dimethylphenyl)-substituted triazatriangulenium (TATA+) cation with various counteranions were synthesized to investigate the interactions for the bulky cation. Single-crystal X-ray analysis of the TATA+ ion pairs revealed solid-state ion-pairing assemblies without stacking at the cationic π-planes. The TATA+ cation showed counteranion-dependent assembly structures, with smaller counteranions located at the top of TATA+ and bulkier counteranions displaced from the TATA+ plane to interact with the surrounding TATA+.
Keywords: charged π-electronic systems; ion pairs; single-crystal X-ray analysis; solid-state assemblies; triazatriangulenium cation.
Copyright © 2024, Haketa et al.
Figures




Similar articles
-
Ion-pairing π-electronic systems: ordered arrangement and noncovalent interactions of negatively charged porphyrins.Chem Sci. 2021 Jun 14;12(28):9645-9657. doi: 10.1039/d1sc02260a. eCollection 2021 Jul 21. Chem Sci. 2021. PMID: 34349936 Free PMC article.
-
Ion-Pairing Assemblies of Polarized Charged Porphyrin.Chemistry. 2025 Sep 1;31(49):e02225. doi: 10.1002/chem.202502225. Epub 2025 Aug 7. Chemistry. 2025. PMID: 40772367 Free PMC article.
-
Ion-Pairing Assemblies Comprising Anion Complexes of π-Extended Anion-Responsive Molecules.J Org Chem. 2019 Jul 19;84(14):8886-8898. doi: 10.1021/acs.joc.9b00754. Epub 2019 Jun 26. J Org Chem. 2019. PMID: 31192598
-
π-Electronic ion pairs: building blocks for supramolecular nanoarchitectonics viaiπ-iπ interactions.Chem Soc Rev. 2023 Oct 16;52(20):7170-7196. doi: 10.1039/d3cs00581j. Chem Soc Rev. 2023. PMID: 37795542 Review.
-
Principles of Cation-π Interactions for Engineering Mussel-Inspired Functional Materials.Acc Chem Res. 2022 Apr 19;55(8):1171-1182. doi: 10.1021/acs.accounts.2c00068. Epub 2022 Mar 28. Acc Chem Res. 2022. PMID: 35344662 Review.
References
-
- Winter A, Schubert U S. Mater Chem Front. 2019;3:2308–2325. doi: 10.1039/c9qm00532c. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
