Enhancing retention and permeation of rapamycin for osteoarthritis therapy using a two-stage drug delivery system
- PMID: 39403313
- PMCID: PMC11471676
- DOI: 10.1016/j.mtbio.2024.101279
Enhancing retention and permeation of rapamycin for osteoarthritis therapy using a two-stage drug delivery system
Abstract
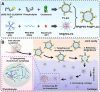
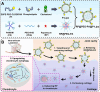
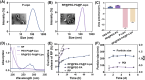
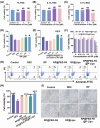
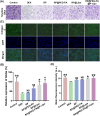
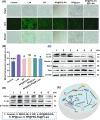
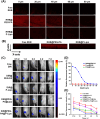
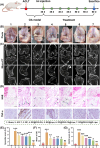
Osteoarthritis (OA) remains a challenging degenerative joint disease, largely associated with chondrocyte apoptosis during its development. Preserving chondrocytes stands as a promising strategy for OA treatment. Rapamycin (RP) exhibits chondrocyte protection by fostering autophagy. Nevertheless, the swift clearance of intra-articular injections and the dense cartilage extracellular matrix (ECM) hinder RP from effectively reaching chondrocytes. Herein, we developed a "two-stage" drug delivery system (RP@PEG-PA@P-Lipo). This system comprises primary nanoparticles (P-Lipo), liposomes modified with a collagen II targeting peptide (WYRGRLC), and secondary nanoparticles (RP@PEG-PA), PEG-modified PAMAM encapsulating rapamycin (RP). RP@PEG-PA@P-Lipo demonstrates adherence to the cartilage surface with WYRGRLC, substantially prolonging retention within the joint cavity. Subsequently, released RP@PEG-PA can effectively penetrate the cartilage and deliver RP to chondrocytes through small size and charge-driven forces. In vitro and in vivo experiments corroborate its notable therapeutic effects on OA. This study holds promise in offering a novel approach for clinical drug delivery and OA treatment.
Keywords: Autophagy; Chondrocyte; Osteoarthritis; Rapamycin; Two-stage nanoparticle.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Chondrocyte-targeted bilirubin/rapamycin carrier-free nanoparticles alleviate oxidative stress and modulate autophagy for osteoarthritis therapy.J Control Release. 2025 Feb 10;378:517-533. doi: 10.1016/j.jconrel.2024.12.024. Epub 2024 Dec 24. J Control Release. 2025. PMID: 39701459
-
ADSCs increase the autophagy of chondrocytes through decreasing miR-7-5p in Osteoarthritis rats by targeting ATG4A.Int Immunopharmacol. 2023 Jul;120:110390. doi: 10.1016/j.intimp.2023.110390. Epub 2023 May 30. Int Immunopharmacol. 2023. PMID: 37262955
-
Intra-articular nanodrug delivery strategies for treating osteoarthritis.Drug Discov Today. 2023 Mar;28(3):103482. doi: 10.1016/j.drudis.2022.103482. Epub 2022 Dec 27. Drug Discov Today. 2023. PMID: 36584875 Review.
-
Parathyroid hormone-(1-34) ameliorated knee osteoarthritis in rats via autophagy.J Appl Physiol (1985). 2018 May 1;124(5):1177-1185. doi: 10.1152/japplphysiol.00871.2017. Epub 2018 Jan 4. J Appl Physiol (1985). 2018. PMID: 29357491
-
Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis.Int J Mol Sci. 2015 Oct 30;16(11):26035-54. doi: 10.3390/ijms161125943. Int J Mol Sci. 2015. PMID: 26528972 Free PMC article. Review.
Cited by
-
Inhibition of mTORC1 by rapamycin results in feedback activation of AktS473 and aggravates hallmarks of osteoarthritis in female mice and non-human primates.bioRxiv [Preprint]. 2025 Mar 6:2024.05.14.594256. doi: 10.1101/2024.05.14.594256. bioRxiv. 2025. PMID: 38798488 Free PMC article. Preprint.
References
-
- Rodriguez-Merchan E.C. Topical therapies for knee osteoarthritis. Postgrad. Med. 2018;130(7):607–612. - PubMed
-
- Arden N.K., Perry T.A., Bannuru R.R., Bruyere O., Cooper C., Haugen I.K., Hochberg M.C., McAlindon T.E., Mobasheri A., Reginster J.Y. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021;17(1):59–66. - PubMed
LinkOut - more resources
Full Text Sources

