Safety and efficacy of CyTisine for smoking cessation in a hOSPital context (CITOSP): study protocol for a prospective observational study
- PMID: 39403432
- PMCID: PMC11471529
- DOI: 10.3389/fpubh.2024.1350176
Safety and efficacy of CyTisine for smoking cessation in a hOSPital context (CITOSP): study protocol for a prospective observational study
Abstract
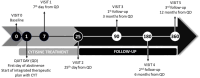
Tobacco addiction is the primary preventable factor contributing to global mortality, and nicotine is one of the substances with the greatest potential for addiction. With a strong affinity for the α4β2 subtype receptor, cytisine (CYT) functions as a partial agonist of the acetylcholine nicotinic cholinergic receptor. It counteracts the effects of nicotine without causing any withdrawal symptoms. These features, combined with its limited mild adverse effects and minimal drug-drug interactions, make cytisine a cost-effective treatment for smoking cessation. The current protocol describes a prospective observational study on the safety and efficacy of CYT administered to inpatient smokers of the Integrated University Hospital of Verona (IUHVR), Veneto (Italy). This is a monocentric, observational, and prospective study on both sex smokers over the age of 18 years admitted to the IUHVR who meet the criteria for recruitment and have given their consent. Eligible participants will be assigned to the CYT intervention based on the West dosing schedule and will be followed up for 12 months from treatment initiation. Evaluation of safety, efficacy, and compliance will be assessed at 7 and 25 days, with follow-up at 3, 6, and 12 months from the start of the treatment (quit day). During each visit, any adverse events or adverse reactions reported by patients following the intake of CYT will be evaluated. This study will contribute, for the first time, to the knowledge about the use of CYT for smoking cessation in a hospital setting.
Keywords: cost-effective; cytisine; hospital setting; protocol; safety; smoking cessation; tobacco addiction.
Copyright © 2024 Torazzi, Tedesco, Ceccato, Santin, Campagnari, Losso, Toldo, Casari, Arzenton, Marini, Chiamulera and Lugoboni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . (2023). WHO report on the global tobacco epidemic: protect people from tobacco smoke. World Health Organization. Available at: https://iris.who.int/handle/10665/372043.
-
- Istituto Superiore della Sanità . Linea guida per il trattamento della dipendenza da tabacco e da nicotina. (2023) Availabe at: https://www.pnrr.salute.gov.it/imgs/C_17_pubblicazioni_3324_allegato.pdf
-
- World Health Organization . (2017) WHO report on the global tobacco epidemic: monitoring tobacco use and prevention policies. World Health Organization. Available at: https://iris.who.int/handle/10665/255874.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical