Validation of a holistic composite outcome measure for the evaluation of chronic pain interventions
- PMID: 39403449
- PMCID: PMC11473062
- DOI: 10.1097/PR9.0000000000001202
Validation of a holistic composite outcome measure for the evaluation of chronic pain interventions
Abstract
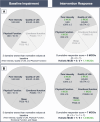
Introduction: Chronic pain is a personal experience influenced by multiple biopsychosocial factors. Using a pain intensity measure alone to assess the effectiveness of a chronic pain intervention fails to fully evaluate its impact on the multifaceted chronic pain experience. The holistic minimal clinically important difference (MCID) is a composite outcome developed to provide a comprehensive assessment of chronic pain in response to intervention, across 5 outcome domains: pain intensity, health-related quality of life, sleep quality, physical, and emotional function. To focus on domains where the individual need is greatest, the holistic MCID reflects the cumulative MCID averaged over only the domains where subjects were impaired preintervention.
Objectives: To assess the internal and construct validity of the Holistic MCID score to inform its future use as an evidence-based tool.
Methods: This validation study was undertaken using data from the EVOKE trial with 111 patients up to 24-month follow-up. Internal consistency of the holistic MCID was assessed using Cronbach alpha statistic and dimensional exploration using principal component analysis.
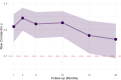
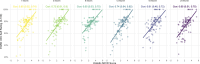
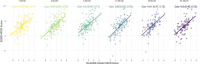
Results: The holistic MCID measure demonstrated strong internal consistency with Cronbach alpha >0.7 at all follow-ups. Principal component analysis showed one overarching holistic dimension to be present in the composite. Construct validity was demonstrated by an increase in the holistic MCID score being associated with both increased Patients' Global Impression of Change, EuroQol visual analogue scale score, and each of the outcome domains in a "leave-one-out" analysis (all P < 0.001).
Conclusion: The holistic MCID provides a valid measure for the comprehensive, personalized assessment of response after a chronic pain intervention. The validity of the holistic MCID requires further confirmation in other chronic pain populations and with different interventions.
Keywords: Chronic pain; Construct validity; Holistic composite outcome measure; Internal consistency; Minimal clinical important difference; Pain measurement.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The International Association for the Study of Pain.
Conflict of interest statement
R.S.T. reports consultancy fees from Medtronic, Nevro and Saluda Medical outside the submitted work. C.M.M. is employed by NAMSA, a company that provides consulting and testing services to medical device manufacturers. N.A.M. reports receiving grants from Neuros, Mesoblast, and Vivex Biologics, as well as consulting as a medical monitor for Saluda Medical, Nevro, Vivex Biologics, Mainstay, Sollis Therapeutics, and Vertos outside the submitted work. J.W.K. is an advisory board member for Boston Scientific, Medtronic, Abbott, and Saluda Medical. J.E.P. reports research and consulting fees from Saluda Medical during the conduct of the study; consultancy for Abbott, Medtronic, Saluda Medical, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, and Thermaquil outside the submitted work; has received grant and research support from: Abbott, Flowonix, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, and Thermaquil outside the submitted work; and is a shareholder of Vertos, SPR Therapeutics, Painteq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil, and Anesthetic Gas Reclamation. C.W.H. has received consultancy fees from Saluda Medical and Genecentrix outside the submitted work. S.J.C. reports receiving grants from Saluda Medical, Vertos, Mainstay, and Vivex outside the submitted work. L.K. reports receiving grants from Nevro, Neuros, Avanos, Medtronic, Neuralace, and Xalud Therapeutics and financial support from Nevro, Avanos, and Saluda Medical outside the submitted work. C.A.G. reports personal fees and other from SPR, and personal fees from Nevro, Nalu, Biotronik, Boston Scientific and Saluda Medical outside the submitted work. E.A.P. has received research support from Mainstay, Medtronic, Neuros Medical, Nevro Corp, ReNeuron, SPR, and Saluda Medical outside the submitted work, as well as personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Presidio Medical, Saluda Medical, and Vertos outside the submitted work. She holds stock options from SynerFuse and neuro42. K.V.P. is a consultant and speaker for Abbott. S.E. reports consultancy fees from Medtronic, and Mainstay Medical outside the submitted work. He has received department research funding from the National Institute of Health Research, Saluda Medical and Medtronic. R.M.L. is an uncompensated consultant for Biotronik, Abbott, Nalu, Saluda Medical, and Mainstay Medical and has stock options from Nalu and Saluda Medical. C.G. reports payment to his institution (for part of his salary) and stock options received from Mainstay, personal fees from Mainstay, Saluda Medical, Persica, and Iliad outside the submitted work, research funded by Sollis, expert witness testimony fees, and serves as Editor-in-Chief of Pain Practice. S.D. has received consulting payments from Averitas Pharma and Biotronik outside the submitted work. A.A. reports consultancy for Medtronic, Avanos, and StimWave outside the submitted work. P.B. reports consulting fees from Abbott and PainTEQ outside the submitted work. E.H., A.L., N.S. and R.V.D. are employees of Saluda Medical. R.V.D. has previously received consultancy fees from Mainstay Medical, Medtronic, and Saluda Medical. D.J.C. has consulted for AbbVie, Heron Therapeutics, Aptinyx, Neumentum, Regeneron Pharmaceuticals, Swing Therapeutics, Virios Therapeutics, Allergan Sales, Eli Lilly and Company, H. Lundback A/S, Pfizer, Samumed, Tonix Pharmaceuticals. T.J.N. has received consultancy fees from Saluda Medical outside the submitted work. The remaining authors declare no conflict of interest.Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48.
-
- Brooks ME, Kristensen K, Benthem K, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 2017;9:378–400.
LinkOut - more resources
Full Text Sources
Medical
