Relationship between dry eye disease and myopia: A systematic review and meta-analysis
- PMID: 39403500
- PMCID: PMC11471511
- DOI: 10.1016/j.heliyon.2024.e38674
Relationship between dry eye disease and myopia: A systematic review and meta-analysis
Abstract
Background/objectives: Dry eye disease (DED) and myopia are common ocular disorders. This systematic review and meta-analysis investigated the association between DED and myopia.
Methods: PubMed and EMBASE were searched for articles published between 1984 and 2022. Study quality was assessed using the Joanna Briggs Institute Critical Appraisal Checklist, and analysis was conducted using the DerSimonian-Laird random-effects model.
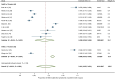
Results: Of the 1,313 studies identified, 15 studies on DED and myopia were included. The meta-analysis revealed that the overall prevalence of subjective DED symptoms in the myopia population was 45.1 % (95 % confidence interval: 0.287-0.616). There was a significant association between DED and myopia. The myopia population had higher Ocular Surface Disease Index scores and shorter tear film breakup times than the non-myopia population. Additionally, the meta-regression analysis showed that spherical equivalent was significantly associated with the prevalence of DED symptoms in adults with myopia.
Conclusion: Interventions to prevent DED are required in the myopia population. Enhancing patient awareness and self-management for DED, in addition to early screening and detection, is especially critical for younger populations who are at a higher risk of developing myopia.
Keywords: Dry eye disease; Meta-analysis; Myopia; On-screen time; Systemaric review; Younger population.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: K.N. and A.M.I. received personal fees from InnoJin, Inc., outside the submitted work. S.N. received consulting fee from Chugai Pharmaceutical Co., Ltd., Kowa Company, Ltd., Novartis Pharma KK., Riverfield Inc., travel reimbursements and speaker fees from Alcon Inc., Boehringer Ingelheim Co., Ltd., Bayer Yakuhin Ltd., Canon Inc., JFC Sales Plan Co., Ltd., Kowa Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Machida Holdings Inc., MSD K.K., Novartis Pharma KK., Novo Nordisk Pharma Ltd., Otsuka Pharmaceutical Co., Ltd., Santen Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd., and Wakamoto Pharmaceutical Co., Ltd. not related to the submitted work. T.I. received non-financial support from Lion Corporation and Sony Network Communications Inc.; grants from Johnson & Johnson Vision Care Inc., Yuimedi Inc., ROHTO Pharmaceutical Co. Ltd., Kobayashi Pharmaceutical Co. Ltd., Kandenko Co. Ltd., and Fukoku Co. Ltd.; and personal fees from Santen Pharmaceutical Co. Ltd., InnoJin Inc., and Ono Pharmaceutical Co., Ltd. not related to the submitted work. The remaining authors declare no competing interests. The sponsors had no role in the design or execution of the study; data collection and management; the analysis and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Craig J.P., et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017;15(3):276–283. - PubMed
LinkOut - more resources
Full Text Sources

