Three doses of Sars-CoV-2 mRNA vaccine in older adults result in similar antibody responses but reduced cellular cytokine responses relative to younger adults
- PMID: 39403561
- PMCID: PMC11472110
- DOI: 10.1016/j.jvacx.2024.100564
Three doses of Sars-CoV-2 mRNA vaccine in older adults result in similar antibody responses but reduced cellular cytokine responses relative to younger adults
Abstract
Objectives: Booster COVID-19 vaccinations are used to protect the elderly, a group vulnerable to severe disease. We compared humoral and cellular immunity in older versus younger adults up to eight months after administering a BNT16b2 booster vaccine dose. Next, we analyzed the plasma levels of soluble T cell activation/exhaustion markers.
Methods: Home-dwelling older adults (n = 68, median age 86) and younger healthcare workers (n = 35, median age 39), previously vaccinated with two doses of BNT162b2, were given a booster dose at ten months after the initial dose. Our analysis consisted of spike-specific IgG, neutralizing antibodies, memory B cells, IFN-γ and IL-2 secreting T cells and soluble T cell exhaustion/activation markers.
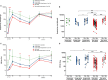
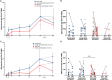
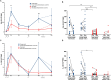
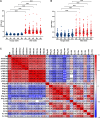
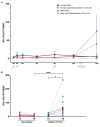
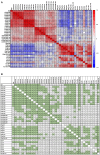
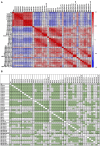
Results: Following the initial two doses, the elderly cohort exhibited lower humoral and IFN-γ responses compared to younger adults. The booster dose increased the humoral responses in both older and younger adults. At two months after the booster dose, older and younger vaccinees had comparable levels of antibodies and the responses were maintained up to 18 months. The younger cohort elicited an increase in the cellular response, while no increase was detected in the elderly. The elderly had higher plasma levels of soluble forms of the T cell activation/exhaustion markers CD25 and TIM-3, which inversely correlated with age and T-cell cytokine responses. This suggests that these markers may be related to the observed dysfunctional cellular cytokine response in older adults. However, both elderly and younger adults who experienced breakthrough infections after booster vaccination, elicited more robust humoral and IFN-γ responses.
Conclusions: The booster dose elicited neutralizing and spike-specific antibody responses in the elderly that were comparable with that of the younger cohort. However, the lack of a strong cellular cytokine response to the third dose in the elderly may explain their vulnerability to severe infection and may be a consequence of exhausted or senescent T cell responses. (https://clinicaltrials.gov/study/NCT04706390).
Keywords: Aging; Interferon-gamma; Memory B cells; Neutralizing antibodies; SARS-CoV-2; T-lymphocytes; mRNA vaccines.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
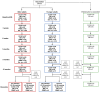


References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

