Screening of photosensitizers-ATP binding cassette (ABC) transporter interactions in vitro
- PMID: 39403604
- PMCID: PMC11472579
- DOI: 10.20517/cdr.2024.50
Screening of photosensitizers-ATP binding cassette (ABC) transporter interactions in vitro
Abstract
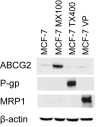
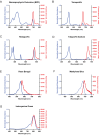
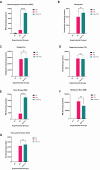
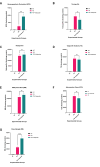
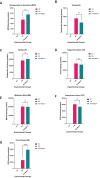
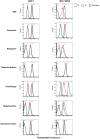
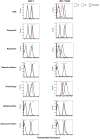
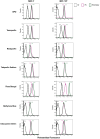
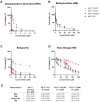
Aim: ATP-binding cassette (ABC) transporters are proteins responsible for the efflux of drug molecules from cancer cells, reducing the efficacy of anti-cancer treatments. This study assesses the susceptibility of a panel of clinically used photosensitizers to be transported by ABC transporters in vitro. Methods: The involvement of P-glycoprotein (P-gp/ABCB1), breast cancer resistance protein (BCRP/ABCG2), and multidrug resistance-associated protein 1 (MRP1/ABCC1) in the transport of 7 clinically utilized photosensitizers [benzoporphyrin derivative (BPD), temoporfin, redaporfin, talaporfin sodium, rose bengal, methylene blue, and indocyanine green] were investigated using human breast cancer cell lines following well-established protocols. Briefly, parental MCF-7 cells and sublines that overexpress P-gp (MCF-7 TX400), ABCG2 (MCF-7 MX100), or MRP1 (MCF-7/VP) were treated with photosensitizers with and without ABC transporter inhibitors. Intracellular levels of photosensitizers were measured using extraction method and flow cytometry to determine whether the ABC transporters are associated with efflux or uptake of photosensitizers. Results: The ABCG2 inhibitor (fumitremorgin C) and P-gp inhibitor (valspodar) effectively blocked the transport mediated by ABCG2 and P-gp of rose bengal and BPD. Redaporfin showed increased accumulation in the presence of valspodar with flow cytometry. Interestingly, MCF-7/VP cells were found to have reduced intracellular accumulation of rose bengal, which was restored with MRP1 inhibitor (MK571). The cell viability assay showed photodynamic therapy (PDT) resistance with Redaporfin in P-gp-overexpressing cells, BPD in ABCG2- and P-gp-overexpressing cells, and with Rose bengal in ABCG2-, P-gp- and MRP1-overexpressing cells, respectively. However, no change in intracellular retention was observed for other photosensitizers. Conclusion: In summary, our study provided new knowledge that temoporfin, talaporfin sodium, methylene blue, and indocyanine green are not substrates of ABCG2, P-gp, or MRP1. Redaporfin is a substrate for P-gp. BPD is a known substrate of ABCG2 and P-gp. Rose bengal is a substrate of ABCG2, P-gp, and MRP1. The results presented here indicate ABC transporter substrate status as a possible cause for cellular resistance to photodynamic therapy with rose bengal, redaporfin, and BPD.
Keywords: ATP-binding cassette (ABC) transporters; Multidrug resistance; photodynamic therapy; photosensitizers.
© The Author(s) 2024.
Conflict of interest statement
Uusimaa P is a co-founder of and has equity in Modulight Corporation, Finland. Huang HC has research agreements with Modulight Corporation, Finland. The remaining authors declared that there are no conflicts of interest.
Figures

References
-
- Obaid G, Celli JP, Broekgaarden M, et al. Engineering photodynamics for treatment, priming and imaging. Nat Rev Bioeng. 2024;2:752–69. doi: 10.1038/s44222-024-00196-z. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
