Discovery of a Small-Molecule Inhibitor Targeting the Biofilm Regulator BrpT in Vibrio vulnificus
- PMID: 39403724
- PMCID: PMC11637837
- DOI: 10.4014/jmb.2406.06052
Discovery of a Small-Molecule Inhibitor Targeting the Biofilm Regulator BrpT in Vibrio vulnificus
Abstract
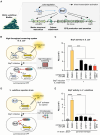
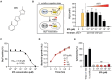
Vibrio vulnificus, an opportunistic human pathogen, employs biofilm formation as a key survival and virulence mechanism. BrpT, a transcriptional regulator, is essential for V. vulnificus biofilm development by regulating the expression of biofilm-related genes. In this study, we aimed to identify a small molecule inhibitor of BrpT to combat V. vulnificus biofilm formation. High-throughput screening of 7,251 compounds using an Escherichia coli reporter strain carrying the arabinose-inducible brpT gene and a BrpT-activated promoter fused to the luxCDABE operon identified a hit compound, BTI (BrpT Inhibitor). BTI potently inhibited BrpT activity in V. vulnificus (EC50 of 6.48 μM) without affecting bacterial growth or host cell viability. Treatment with BTI significantly reduced the expression of the BrpT regulon and impaired biofilm formation and colony rugosity in V. vulnificus, thus increasing its susceptibility to antibiotics. In vitro biochemical analyses revealed that BTI directly binds to BrpT and inhibits its transcriptional regulatory activity. The identification of BTI as a specific inhibitor of BrpT that effectively diminishes V. vulnificus biofilm formation provides a promising foundation for the development of novel anti-biofilm strategies, with the potential to address the growing challenge of antibiotic resistance and improve the treatment of biofilm-associated infections.
Keywords: BrpT; Vibrio vulnificus; biofilm; high-throughput screening; small-molecule inhibitor; transcription regulation.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures


Similar articles
-
Complex Control of a Genomic Island Governing Biofilm and Rugose Colony Development in Vibrio vulnificus.J Bacteriol. 2018 Jul 25;200(16):e00190-18. doi: 10.1128/JB.00190-18. Print 2018 Aug 15. J Bacteriol. 2018. PMID: 29760209 Free PMC article.
-
Two novel genes identified by large-scale transcriptomic analysis are essential for biofilm and rugose colony development of Vibrio vulnificus.PLoS Pathog. 2023 Jan 19;19(1):e1011064. doi: 10.1371/journal.ppat.1011064. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36656902 Free PMC article.
-
Identification and Validation of an Antivirulence Agent Targeting HlyU-Regulated Virulence in Vibrio vulnificus.Front Cell Infect Microbiol. 2018 May 11;8:152. doi: 10.3389/fcimb.2018.00152. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868508 Free PMC article.
-
Complex regulatory networks of virulence factors in Vibrio vulnificus.Trends Microbiol. 2022 Dec;30(12):1205-1216. doi: 10.1016/j.tim.2022.05.009. Epub 2022 Jun 23. Trends Microbiol. 2022. PMID: 35753865 Review.
-
Molecular Pathogenesis of Vibrio vulnificus.J Microbiol. 2005 Feb;43 Spec No:118-31. J Microbiol. 2005. PMID: 15765065 Review.
Cited by
-
Metabolic profiling and genetic tool development in the mucosal bacterium Selenomonas sputigena.Genes Genomics. 2025 Aug 20. doi: 10.1007/s13258-025-01668-1. Online ahead of print. Genes Genomics. 2025. PMID: 40835787
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

