Transient transition from Stable to Dissipative Assemblies in Response to the Spatiotemporal Availability of a Chemical Fuel
- PMID: 39403854
- PMCID: PMC11720371
- DOI: 10.1002/anie.202414495
Transient transition from Stable to Dissipative Assemblies in Response to the Spatiotemporal Availability of a Chemical Fuel
Abstract
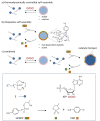
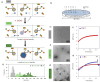
The transition from inactive to active matter implies a transition from thermodynamically stable to energy-dissipating structures. Here, we show how the spatiotemporal availability of a chemical fuel causes a thermodynamically stable self-assembled structure to transiently pass to an energy-dissipating state. The system relies on the local injection of a weak affinity phosphodiester substrate into an agarose hydrogel containing surfactant-based structures templated by ATP. Injection of substrate leads to the inclusion of additional surfactant molecules in the assemblies leading to the formation of catalytic hotspots for substrate conversion. After the local disappearance of the substrate as a result of chemical conversion and diffusion the assemblies spontaneously return to the stable state, which can be reactivated upon the injection of a new batch of fuel. The study illustrates how a dissipating self-assembled system can cope with the intermittent availability of chemical energy without compromising long-term structural stability.
Keywords: active matter; dissipative self-assembly; hydrogel; reaction-diffusion; systems chemistry.
© 2024 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

