Patient-reported data on the severity of Von Willebrand disease
- PMID: 39403864
- PMCID: PMC11659499
- DOI: 10.1111/hae.15103
Patient-reported data on the severity of Von Willebrand disease
Abstract
Introduction: The severity of Von Willebrand disease (VWD) is currently based on laboratory phenotype. However, little is known about the severity of the patient's experience with the disease. The most recent VWD guidelines highlight the need for patient-reported outcomes (PROs) in VWD.
Aim: The study aimed to investigate the patient-perspective on VWD severity and to identify key factors that determine the severity of disease experienced by patients.
Materials and methods: Patients participated in a nationwide cross-sectional study on VWD in the Netherlands (WiN-study). Patients filled in a questionnaire containing questions on the experienced severity of VWD (4-point scale), bleeding score (BS) and quality of life (QoL).
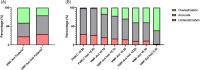
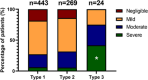
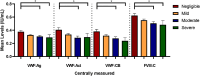
Results: We included 736 patients, median age of 41.0 years (IQR 23.0-55.0) and 59.5% were women. A total of 443 had type 1, 269 type 2 and 24 type 3 VWD. Self-reported severity of VWD was categorized as severe (n = 52), moderate (n = 171), mild (n = 393) or negligible (n = 120). Classification by historically lowest FVIII:C levels < 0.20 IU/mL as a proxy for severe VWD aligned with patient-reported severity classification with a 72% accuracy. Type 3 VWD (OR = 4.02, 95%CI: 1.72-9.45), higher BS (OR = 1.09, 95%CI: 1.06-1.11), female sex (OR = 1.36, 95%CI: 1.01-1.83), haemostatic treatment in the year preceding study inclusion (OR = 1.53, 95%CI: 1.10-2.13) and historically lowest VWF:Act levels (OR = 0.26, 95%CI: 0.07-1.00) were independent determinants of patient-reported severity.
Conclusion: This study shows that patient-reported data provide novel insights into the determinants of experienced disease severity. Our findings highlight the need for studies on PROs with validated questionnaires to assess the burden of VWD.
Keywords: Von Willebrand disease; classification; disease severity; patient reported outcome measures; quality of life.
© 2024 The Author(s). Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
Ferdows Atiq is supported by a Rubicon grant (452022310) from the Netherlands Organization for Health Research and Development (ZonMw). Ferdows Atiq received research support from CSL Behring, Takeda, Octapharma and Sobi. Marjon H. Cnossen has received grants from governmental research institutes, such as the Dutch Research Institute (NWO), ZonMW, Innovation Fund, NWO‐Dutch Research Agenda, and unrestricted investigator‐initiated research grants as well as educational and travel funding from various companies over the years (Pfizer, Baxter/Baxalta/Shire, Bayer Schering Pharma, CSL Behring, Sobi, Novo Nordisk, Novartis, and Nordic Pharma); she served as a member on steering boards of Roche and Bayer. All grants, awards, and fees go to the Erasmus MC. Karina Meijer reports speaker fees from Alexion, Bayer and CSL Behring, participation in trial steering committees for Bayer and Astra Zeneca, consulting fees from Uniqure and Therini, participation in data monitoring and endpoint adjudication committee for Octapharma. Marieke J.H.A. Kruip received grants from governmental research institutes, such as the Dutch Research Institute (ZonMW/NWO), Dutch Thrombosis Foundation, Innovation fund, unrestricted grants from Bayer, Pfizer, Daiichi Sankyo, Sobi and Boehringer Ingelheim and speakers fee from Bayer. Jeroen Eikenboom received research support from CSL Behring. Karin P.M. van Galen received unrestricted research support from Octapharma. Frank W.G. Leebeek received unrestricted research support from CSL Behring and Takeda for performing the Willebrand in the Netherlands (WiN) study and uniQure and SOBI for studies not related to this article, and he is a consultant for uniQure, CSL Behring, BioMarin, and Takeda, of which the fees go to the institution. Calvin B. van Kwawegen, Karin Fijnvandraat, Saskia E.M. Schols, Joke de Meris and Johanna G. van der Bom declare no conflicts of interest.
Figures

References
-
- Leebeek FW, Eikenboom JC. Von Willebrand's Disease. N Engl J Med. 2016;375(21):2067‐2080. - PubMed
-
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of Von Willebrand disease: a report of the Subcommittee on Von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103‐2114. - PubMed
-
- Federici AB. Clinical diagnosis of Von Willebrand disease. Haemophilia. 2004;10(4):169‐176. - PubMed
-
- Nichols WL, Hultin MB, James AH, et al. Von Willebrand disease (VWD): evidence‐based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171‐232. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

