AGO2 protein: a key enzyme in the miRNA pathway as a novel biomarker in adrenocortical carcinoma
- PMID: 39404265
- PMCID: PMC11623120
- DOI: 10.1530/ERC-24-0061
AGO2 protein: a key enzyme in the miRNA pathway as a novel biomarker in adrenocortical carcinoma
Abstract
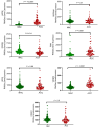
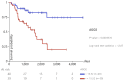
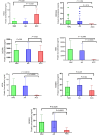
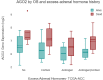
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy characterized by diagnostic challenges, high recurrence rates, and poor prognosis. This study explored the role of miRNA processing genes in ACC and their potential role as diagnostic and prognostic biomarkers. We analyzed the mRNA expression levels of miRNA machinery components (DROSHA, DGCR8, XPO5, RAN, DICER, TARBP2, and AGO2) utilizing mRNA-Seq data from The Cancer Genome Atlas (TCGA) and The Genotype-Tissue Expression (GTEx) projects. Additionally, protein levels were quantified in tissue samples from the Kolling Institute of Medical Research's tumor bank. Our results demonstrated that among all miRNA processing components, AGO2 exhibited significant overexpression in ACC compared to the normal adrenal cortex and benign adrenal adenoma (P < 0.001). Kaplan-Meier survival analysis indicated that higher AGO2 expression correlated with significantly worse overall survival in ACC patients (HR: 7.07, P < 0.001). Among 32 cancer types in TCGA, the prognostic significance of AGO2 was most prominent in ACC. This study is the first to report AGO2's potential as a diagnostic and prognostic biomarker in ACC, emphasizing its significance in ACC pathogenesis and potential application as a non-invasive liquid biopsy biomarker.
Keywords: AGO2-protein; adrenocortical carcinoma; diagnostic and prognostic biomarkers; miRNA.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Figures


References
-
- Amini N, Margonis GA, Kim Y, Tran TB, Postlewait LM, Maithel SK, Wang TS, Evans DB, Hatzaras I, Shenoy R, et al. 2016. Curative resection of adrenocortical carcinoma: rates and patterns of postoperative recurrence. Annals of Surgical Oncology 23 126–133. ( 10.1245/s10434-015-4810-y) - DOI - PMC - PubMed
-
- Bartha Á & Győrffy B. 2021. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. International Journal of Molecular Sciences 22 2622. ( 10.3390/ijms22052622) - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

