Investigation into the Simulation and Mechanisms of Metal-Organic Framework Membrane for Natural Gas Dehydration
- PMID: 39404310
- PMCID: PMC11478295
- DOI: 10.3390/nano14191583
Investigation into the Simulation and Mechanisms of Metal-Organic Framework Membrane for Natural Gas Dehydration
Abstract
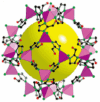
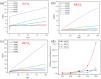
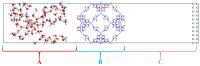
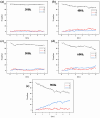
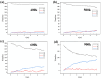
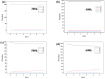
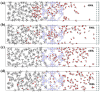
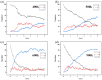
Natural gas dehydration is a critical process in natural gas extraction and transportation, and the membrane separation method is the most suitable technology for gas dehydration. In this paper, based on molecular dynamics theory, we investigate the performance of a metal-organic composite membrane (ZIF-90 membrane) in natural gas dehydration. The paper elucidates the adsorption, diffusion, permeation, and separation mechanisms of water and methane with the ZIF-90 membrane, and clarifies the influence of temperature on gas separation. The results show that (1) the diffusion energy barrier and pore size are the primary factors in achieving the separation of water and methane. The diffusion energy barriers for the two molecules (CH4 and H2O) are ΔE(CH4) = 155.5 meV and ΔE(H2O) = 50.1 meV, respectively. (2) The ZIF-90 is more selective of H2O, which is mainly due to the strong interaction between the H2O molecule and the polar functional groups (such as aldehyde groups) within the ZIF-90. (3) A higher temperature accelerates the gas separation process. The higher the temperature is, the faster the separation process is. (4) The pore radius is identified as the intrinsic mechanism enabling the separation of water and methane in ZIF-90 membranes.
Keywords: mechanism; metal–organic composite membrane; molecular dynamics simulation; natural gas dehydration; temperature.
Conflict of interest statement
Author Pengxiao Liu was employed by the company PetroChina Tarim Oilfield Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Controlled Covalent Functionalization of ZIF-90 for Selective CO2 Capture & Separation.Membranes (Basel). 2022 Oct 27;12(11):1055. doi: 10.3390/membranes12111055. Membranes (Basel). 2022. PMID: 36363610 Free PMC article.
-
Cellulose acetate-based membranes by interfacial engineering and integration of ZIF-62 glass nanoparticles for CO2 separation.J Hazard Mater. 2021 Aug 5;415:125639. doi: 10.1016/j.jhazmat.2021.125639. Epub 2021 Mar 13. J Hazard Mater. 2021. PMID: 33740720
-
Influence of ligands within Al-based metal-organic frameworks for selective separation of methane from unconventional natural gas.Chemosphere. 2023 Apr;321:138160. doi: 10.1016/j.chemosphere.2023.138160. Epub 2023 Feb 14. Chemosphere. 2023. PMID: 36796522
-
Tuning the Phase Composition of Metal-Organic Framework Membranes for Helium Separation through Incorporation of Fullerenes.J Am Chem Soc. 2023 Jul 12;145(27):14793-14801. doi: 10.1021/jacs.3c03362. Epub 2023 Jun 23. J Am Chem Soc. 2023. PMID: 37351897 Free PMC article.
-
A Review on Mixed Matrix Membranes for Solvent Dehydration and Recovery Process.Membranes (Basel). 2021 Jun 11;11(6):441. doi: 10.3390/membranes11060441. Membranes (Basel). 2021. PMID: 34208292 Free PMC article. Review.
References
-
- Economides M.J., Wood D.A. The state of natural gas. J. Nat. Gas Sci. Eng. 2009;1:1–13. doi: 10.1016/j.jngse.2009.03.005. - DOI
-
- Stanek W., Białecki R. Can natural gas warm the climate more than coal? Fuel. 2014;136:341–348. doi: 10.1016/j.fuel.2014.07.075. - DOI
-
- Chebbi R., Qasim M., Jabbar N.A. Optimization of triethylene glycol dehydration of natural gas. Energy Rep. 2019;5:723–732. doi: 10.1016/j.egyr.2019.06.014. - DOI
-
- Liu S., Zhang Y., Sun Z., Wang Z. Research Progress in Natural Gas Dehydration Technologies. Chem. Eng. Mach. 2022;49:574–580.
-
- Chen J., Ma W., Zhao P., Wei A., Zhang L. Development status and trend of natural gas dehydration process. Petrochem. Ind. Appl. 2023;42:8–10.
Grants and funding
LinkOut - more resources
Full Text Sources

