Treadmill Exercise Facilitates Synaptic Plasticity in APP/PS1 Mice by Regulating Hippocampal AMPAR Activity
- PMID: 39404372
- PMCID: PMC11475322
- DOI: 10.3390/cells13191608
Treadmill Exercise Facilitates Synaptic Plasticity in APP/PS1 Mice by Regulating Hippocampal AMPAR Activity
Abstract
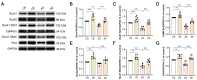
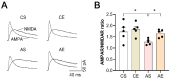
Accumulating evidence underscores exercise as a straightforward and cost-effective lifestyle intervention capable of mitigating the risk and slowing the emergence and progression of Alzheimer's disease (AD). However, the intricate cellular and molecular mechanisms mediating these exercise-induced benefits in AD remain elusive. The present study delved into the impact of treadmill exercise on memory retrieval performance, hippocampal synaptic plasticity, synaptic morphology, and the expression and activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptors (AMPARs) in 6-month-old APP/PS1 mice. APP/PS1 mice (4-month-old males) were randomly assigned to either a treadmill exercise group or a sedentary group, with C57BL/6J mice (4-month-old males) as the control group (both exercise and sedentary). The exercise regimen spanned 8 weeks. Our findings revealed that 8-week treadmill exercise reversed memory retrieval impairment in step-down fear conditioning in 6-month-old APP/PS1 mice. Additionally, treadmill exercise enhanced basic synaptic strength, short-term potentiation (STP), and long-term potentiation (LTP) of the hippocampus in these mice. Moreover, treadmill exercise correlated with an augmentation in synapse numbers, refinement of synaptic structures, and heightened expression and activity of AMPARs. Our findings suggest that treadmill exercise improves behavioral performance and facilitates synaptic transmission by increasing structural synaptic plasticity and the activity of AMPARs in the hippocampus of 6-month-old APP/PS1 mice, which is involved in pre- and postsynaptic processes.
Keywords: AMPA receptor; Alzheimer’s disease; exercise; synaptic plasticity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

