The Role of Non-Coding RNAs in Regulating Cachexia Muscle Atrophy
- PMID: 39404384
- PMCID: PMC11482569
- DOI: 10.3390/cells13191620
The Role of Non-Coding RNAs in Regulating Cachexia Muscle Atrophy
Abstract
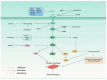
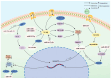
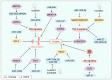
Cachexia is a late consequence of various diseases that is characterized by systemic muscle loss, with or without fat loss, leading to significant mortality. Multiple signaling pathways and molecules that increase catabolism, decrease anabolism, and interfere with muscle regeneration are activated. Non-coding RNAs (ncRNAs), such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), play vital roles in cachexia muscle atrophy. This review mainly provides the mechanisms of specific ncRNAs to regulate muscle loss during cachexia and discusses the role of ncRNAs in cachectic biomarkers and novel therapeutic strategies that could offer new insights for clinical practice.
Keywords: cachexia; circRNAs; lncRNAs; microRNAs; muscle atrophy; non-coding RNAs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Noncoding RNAs in Muscle Atrophy.Adv Exp Med Biol. 2018;1088:249-266. doi: 10.1007/978-981-13-1435-3_11. Adv Exp Med Biol. 2018. PMID: 30390255 Review.
-
Exploring the Regulatory Landscape of Dementia: Insights from Non-Coding RNAs.Int J Mol Sci. 2024 Jun 4;25(11):6190. doi: 10.3390/ijms25116190. Int J Mol Sci. 2024. PMID: 38892378 Free PMC article. Review.
-
Emerging Role of Non-Coding RNAs in Esophageal Squamous Cell Carcinoma.Int J Mol Sci. 2019 Dec 30;21(1):258. doi: 10.3390/ijms21010258. Int J Mol Sci. 2019. PMID: 31905958 Free PMC article. Review.
-
Noncoding RNA crosstalk in brain health and diseases.Neurochem Int. 2021 Oct;149:105139. doi: 10.1016/j.neuint.2021.105139. Epub 2021 Jul 16. Neurochem Int. 2021. PMID: 34280469 Free PMC article. Review.
-
Noncoding RNAs, Emerging Regulators of Skeletal Muscle Development and Diseases.Biomed Res Int. 2015;2015:676575. doi: 10.1155/2015/676575. Epub 2015 Jul 14. Biomed Res Int. 2015. PMID: 26258142 Free PMC article. Review.
Cited by
-
Circulating miRNAs Signature as a Predictor of Cachexia in Chronic Heart Failure: Diagnostic and Prognostic Implications.J Cardiovasc Transl Res. 2025 Jul 10. doi: 10.1007/s12265-025-10658-3. Online ahead of print. J Cardiovasc Transl Res. 2025. PMID: 40637997
-
Myokines and Microbiota: New Perspectives in the Endocrine Muscle-Gut Axis.Nutrients. 2024 Nov 25;16(23):4032. doi: 10.3390/nu16234032. Nutrients. 2024. PMID: 39683426 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 104004092 and 104004460/the Research Council of the University of Hong Kong
- 200007008/the Gaia Family Trust of New Zealand
- 740608, 766211, 17152116 and 17121419/the Research Grants Committee (RGC) of Hong Kong, HKSAR
- 15162961, 16172751 and 18192141/Health and Medical Research Fund
- 204610519/Enhanced new staff start-up fund
LinkOut - more resources
Full Text Sources

