Berberine Inhibits the Inflammatory Response Induced by Staphylococcus aureus Isolated from Atopic Eczema Patients via the TNF-α/Inflammation/RAGE Pathways
- PMID: 39404402
- PMCID: PMC11475634
- DOI: 10.3390/cells13191639
Berberine Inhibits the Inflammatory Response Induced by Staphylococcus aureus Isolated from Atopic Eczema Patients via the TNF-α/Inflammation/RAGE Pathways
Abstract
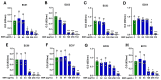
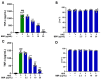
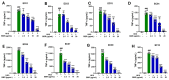
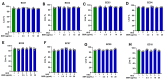
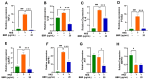
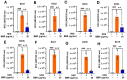
Atopic eczema patients exhibit high levels of Staphylococcus aureus (S. aureus) skin colonization. S. aureus can stimulate macrophages and the expression of proinflammatory cytokines. Berberine (BBR), an alkaloid, attenuates S. aureus toxin production. This study investigated if BBR suppressed bacterial growth and inflammatory response induced by eczema-patient-derived S. aureus using murine macrophage (RAW 264.7) and human monocyte cell lines (U937). RAW 264.7 and U937 were treated with BBR at different concentrations and stimulated with heat-killed S. aureus (ATCC #33591) or S. aureus derived from severe eczema patients (EC01-EC10), who were undergoing topical steroid withdrawal, for 24 h. TNF-α protein levels were determined by ELISA, gene expression by qRT-PCR, cell cytotoxicity by trypan blue excursion, and reactive oxygen species (ROS) levels by fluorometric assay. BBR showed a bacteriostatic effect in S. aureus (ATCC strain #33591 and clinical isolates (EC01-EC10) and suppressed TNF-α production in RAW 264.7 and U937 cells exposed to heat-killed S. aureus (ATCC and clinical isolates) dose-dependently without any cell cytotoxicity. BBR (20 µg/mL) suppressed >90% of TNF-α production (p < 0.001), downregulated genes involved in inflammatory pathways, and inhibited S. aureus ROS production in U937 and RAW 264.7 cells (p < 0.01). BBR suppresses S. aureus-induced inflammation via inhibition of TNF-α release, ROS production, and expression of key genes involved in the inflammatory pathway.
Keywords: S. aureus; TNF-α; atopic dermatitis; berberine; inflammation.
Conflict of interest statement
X-M Li received research support to her institution from the National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM) # 1P01 AT002644725-01“Center for Chinese Herbal Therapy 320 (CHT) for Asthma”, and grant #1R01AT001495-01A1 and 2R01 AT001495-05A2, NIH/NIAID R43AI148039, NIH/NIAID 1R21AI176061-01, NIH/NIAID 1R44AI177183-01, NIH/NIAID 1R41AI172572-01A1, Food Allergy Research and Education (FARE), Winston Wolkoff Integrative Medicine Fund for Allergies and Wellness, the Parker Foundation and Henan University of Chinese Medicine, the Study of Integrative Medicine, the Lie-Artati Family Fund; received consultancy fees from FARE and Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Bayer Global Health LLC; received royalties from UpToDate; share US patent US7820175B2 (FAHF-2), US10500169B2 (XPP), US10406191B2 (S. Flavescens), US10028985B2 (WL); US11351157B2 (nanoBBR); take compensation from her practice at Center for Integrative Health and Acupuncture PC; US Times Technology Inc is managed by her related party; is a member of General Nutraceutical Technology LLC. Nan Yang received research support for his institute from the National Institutes of Health (NIH)/ National Center for Complementary and Alternative Medicine (NCCAM), NIH/NIAID R43AI148039, NIH/NIAID 1R21AI176061-01, NIH/NIAID 1R44AI177183-01, NIH/NIAID 1R41AI172572-01A1; shares US patents US10500169B2 (XPP), US10406191B2 (S. flavescens), US10028985B2 (WL); is a member of General Nutraceutical Technology, LLC; receives a salary from General Nutraceutical Technology, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Liu H., Archer N.K., Dillen C.A., Wang Y., Ashbaugh A.G., Ortines R.V., Kao T., Lee S.K., Cai S.S., Miller R.J., et al. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe. 2017;22:653–666.e655. doi: 10.1016/j.chom.2017.10.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

