Kisspeptin Alleviates Human Hepatic Fibrogenesis by Inhibiting TGFβ Signaling in Hepatic Stellate Cells
- PMID: 39404414
- PMCID: PMC11476267
- DOI: 10.3390/cells13191651
Kisspeptin Alleviates Human Hepatic Fibrogenesis by Inhibiting TGFβ Signaling in Hepatic Stellate Cells
Abstract
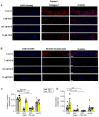
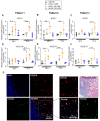
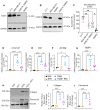
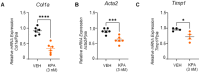
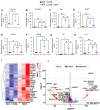
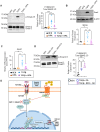
The peptide hormone kisspeptin attenuates liver steatosis, metabolic dysfunction-associated steatohepatitis (MASH), and fibrosis in mouse models by signaling via the kisspeptin 1 receptor (KISS1R). However, whether kisspeptin impacts fibrogenesis in the human liver is not known. We investigated the impact of a potent kisspeptin analog (KPA) on fibrogenesis using human precision-cut liver slices (hPCLS) from fibrotic livers from male patients, in human hepatic stellate cells (HSCs), LX-2, and in primary mouse HSCs. In hPCLS, 48 h and 72 h of KPA (3 nM, 100 nM) treatment decreased collagen secretion and lowered the expression of fibrogenic and inflammatory markers. Immunohistochemical studies revealed that KISS1R is expressed and localized to HSCs in MASH/fibrotic livers. In HSCs, KPA treatment reduced transforming growth factor b (TGFβ)-the induced expression of fibrogenic and inflammatory markers, in addition to decreasing TGFβ-induced collagen secretion, cell migration, proliferation, and colony formation. Mechanistically, KISS1R signaling downregulated TGFβ signaling by decreasing SMAD2/3 phosphorylation via the activation of protein phosphatases, PP2A, which dephosphorylates SMAD 2/3. This study revealed for the first time that kisspeptin reverses human hepatic fibrogenesis, thus identifying it as a new therapeutic target to treat hepatic fibrosis.
Keywords: KISS1R; MASH; MASLD; TGFβ; fibrosis; hepatic stellate cells; kisspeptin.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. MB is a coinventor on a patent application US20230256051A1 filed by Rutgers University. S.L.F has relationships with the companies listed below; however, these activities are unrelated to the content of this article: Consulting: 89 Bio, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, ChemomAb, Foresite Laboratories, Gordian Biotechnology, Glycotest, Glympse Bio, Hepgene, In sitro, Junevity, Korro Bio, Kriya, Laekna, Lerna Therapeutics, Macomics, Mediar, Merck, Morphic Therapeutics, North Sea Therapeutics, Ochre Bio, Overtone Therapeutics, Pfizer Pharmaceuticals, Pliant, Prosciento, RAPT, Sagimet, Satellite Bio, Seal Rock, Scholar Rock, Sunbird Bio, Surrozen, Takeda, Variant Bio. Stock options: Escient, Galectin, Galmed, Genfit, Gordian Biotechnology, Hepgene, Junevity, Lifemax, Metacrine, Morphic Therapeutics, Nimbus, North Sea, Ochre Bio, Therapeutics, Scholar Rock, and Sunbird Bio. Research Activities with Commercial Entities: Abalone Bio (SBIR Grant) and Novo Nordisk. The other authors declare no conflicts of interest that pertain to this work.
Figures




References
-
- Llovet J.M., Willoughby C.E., Singal A.G., Greten T.F., Heikenwalder M., El-Serag H.B., Finn R.S., Friedman S.L. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 2023;20:487–503. doi: 10.1038/s41575-023-00754-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

