The Neolignan Honokiol and Its Synthetic Derivative Honokiol Hexafluoro Reduce Neuroinflammation and Cellular Senescence in Microglia Cells
- PMID: 39404415
- PMCID: PMC11482602
- DOI: 10.3390/cells13191652
The Neolignan Honokiol and Its Synthetic Derivative Honokiol Hexafluoro Reduce Neuroinflammation and Cellular Senescence in Microglia Cells
Abstract
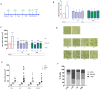
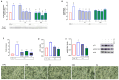
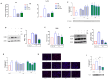
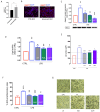
Microglia-mediated neuroinflammation has been linked to neurodegenerative disorders. Inflammation and aging contribute to microglial senescence. Microglial senescence promotes the development of neurodegenerative disorders, including Alzheimer's disease (AD). In this study, we investigated the anti-neuroinflammatory and anti-senescence activity of Honokiol (HNK), a polyphenolic neolignane from Magnolia officinalis Rehder & E.H Wilson, in comparison with its synthetic analogue Honokiol Hexafluoro (CH). HNK reduced the pro-inflammatory cell morphology of LPS-stimulated BV2 microglia cells and increased the expression of the anti-inflammatory cytokine IL-10 with an efficacy comparable to CH. HNK and CH were also able to attenuate the alterations in cell morphology associated with cellular senescence in BV2 cells intermittently stimulated with LPS and significantly reduce the activity and expression of the senescence marker ß-galactosidase and the expression of p21 and pERK1/2. The treatments reduced the expression of senescence-associated secretory phenotype (SASP) factors IL-1ß and NF-kB, decreased ROS production, and abolished H2AX over phosphorylation (γ-H2AX) and acetylated H3 overexpression. Senescent microglia cells showed an increased expression of the Notch ligand Jagged1 that was reduced by HNK and CH with a comparable efficacy to the Notch inhibitor DAPT. Overall, our data illustrate a protective activity of HNK and CH on neuroinflammation and cellular senescence in microglia cells involving a Notch-signaling-mediated mechanism and suggesting a potential therapeutic contribution in aging-related neurodegenerative diseases.
Keywords: Claisened Hexafluoro; Honokiol; Notch signaling; cellular senescence; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

