Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia
- PMID: 39404420
- PMCID: PMC11475131
- DOI: 10.3390/cells13191657
Adenosine Metabolism Pathway Alterations in Frontal Cortical Neurons in Schizophrenia
Abstract
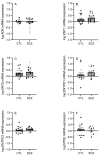
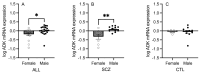
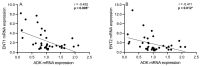
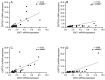
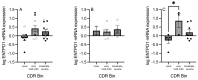
Schizophrenia is a neuropsychiatric illness characterized by altered neurotransmission, in which adenosine, a modulator of glutamate and dopamine, plays a critical role that is relatively unexplored in the human brain. In the present study, postmortem human brain tissue from the anterior cingulate cortex (ACC) of individuals with schizophrenia (n = 20) and sex- and age-matched control subjects without psychiatric illness (n = 20) was obtained from the Bronx-Mount Sinai NIH Brain and Tissue Repository. Enriched populations of ACC pyramidal neurons were isolated using laser microdissection (LMD). The mRNA expression levels of six key adenosine pathway components-adenosine kinase (ADK), equilibrative nucleoside transporters 1 and 2 (ENT1 and ENT2), ectonucleoside triphosphate diphosphohydrolases 1 and 3 (ENTPD1 and ENTPD3), and ecto-5'-nucleotidase (NT5E)-were quantified using real-time PCR (qPCR) in neurons from these individuals. No significant mRNA expression differences were observed between the schizophrenia and control groups (p > 0.05). However, a significant sex difference was found in ADK mRNA expression, with higher levels in male compared with female subjects (Mann-Whitney U = 86; p < 0.05), a finding significantly driven by disease (t(17) = 3.289; p < 0.05). Correlation analyses also demonstrated significant associations (n = 12) between the expression of several adenosine pathway components (p < 0.05). In our dementia severity analysis, ENTPD1 mRNA expression was significantly higher in males in the "mild" clinical dementia rating (CDR) bin compared with males in the "none" CDR bin (F(2, 13) = 5.212; p < 0.05). Lastly, antipsychotic analysis revealed no significant impact on the expression of adenosine pathway components between medicated and non-medicated schizophrenia subjects (p > 0.05). The observed sex-specific variations and inter-component correlations highlight the value of investigating sex differences in disease and contribute to the molecular basis of schizophrenia's pathology.
Keywords: adenosine kinase; anterior cingulate cortex; ecto-5′-nucleotidases; ectonucleoside triphosphate diphosphohydrolases; equilibrative nucleoside transporters; neuromodulation; pyramidal neurons; schizophrenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
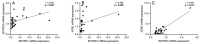

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

