Development of a safe and broad-spectrum attenuated PEDV vaccine candidate by S2 subunit replacement
- PMID: 39404450
- PMCID: PMC11575183
- DOI: 10.1128/jvi.00429-24
Development of a safe and broad-spectrum attenuated PEDV vaccine candidate by S2 subunit replacement
Abstract
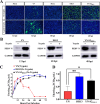
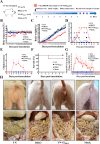
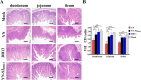
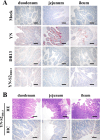
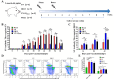
Porcine epidemic diarrhea (PED) has caused serious economic losses to the swine livestock industry. Due to the rapid variation in the PEDV) genome, especially the spike (S) protein, the cross-protection ability of antibodies between different vaccine strains is weakened. Hence, the rapid development of safe, broad-spectrum and highly effective attenuated PEDV vaccine still needs further research. Here, we found that the replacement of the S2 subunit had little effect on S protein immunogenicity. Moreover, the chimeric virus (YN-S2DR13), the S protein of the YN strain was replaced by the DR13 S2 subunit, which lost its trypsin tropism and increased its propagation ability (approximately 1 titer) in Vero cells. Then, the pathogenesis of YN-S2DR13 was evaluated in neonatal piglets. Importantly, quantitative real-time PCR, histopathology, and immunohistochemistry confirmed that the virulence of YN-S2DR13 was significantly reduced compared with that of YN. Immunization with YN-S2DR13 induced neutralizing antibodies against both YN and DR13 in weaned piglets. In vitro passaging data also showed that YN-S2DR13 had good genetic stability. Collectively, these results suggest that YN-S2DR13 has significant advantages as a novel vaccine candidate, including a capacity for viral propagation to high titers with no trypsin requirement and the potential to provide protection against both PEDV G1 and G2 strains infections. Our results also suggests that S2 subunit replacement using reverse genetics can be a rapid strategy for the rational design of live attenuated vaccines for PEDV.
Importance: Emerging highly virulent porcine epidemic diarrhea virus (PEDV) G2 strains has caused substantial economic losses worldwide. Vaccination with a live attenuated vaccine is a promising method to prevent and control PED because it can induce a strong immune response (including T- and B-cell immunity). Previous studies have demonstrated that the S2 subunit of the PEDV spike (S) protein is the determinant of PEDV trypsin independence. Here, we evaluated the pathogenicity, tissue tropism, and immunogenicity of the chimeric virus (YN-S2DR13) via animal experiments. We demonstrated that YN-S2DR13 strain, as a trypsin independent strain, increased intracellular proliferation capacity, significantly reduced virulence, and induced broad-spectrum neutralization protection against PEDV G1 and G2 strains. In vitro passaging data also validated the stability of YN-S2DR13. Our results showed that generating a chimeric PEDV strain that is trypsin-independent by replacing the S2 subunit is a promising approach for designing a live attenuated vaccine for PEDV in the future.
Keywords: chimeric virus; immunogenicity; porcine epidemic diarrhea virus (PEDV); spike protein; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. 2013. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest 25:649–654. doi: 10.1177/1040638713501675 - DOI - PubMed
-
- Liu X, Zhang L, Zhang Q, Zhou P, Fang Y, Zhao D, Feng J, Li W, Zhang Y, Wang Y. 2019. Evaluation and comparison of immunogenicity and cross-protective efficacy of two inactivated cell culture-derived GIIa- and GIIb-genotype porcine epidemic diarrhea virus vaccines in suckling piglets. Vet Microbiol 230:278–282. doi: 10.1016/j.vetmic.2019.02.018 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

