Illuminating cellular architecture and dynamics with fluorescence polarization microscopy
- PMID: 39404619
- PMCID: PMC11529880
- DOI: 10.1242/jcs.261947
Illuminating cellular architecture and dynamics with fluorescence polarization microscopy
Abstract
Ever since Robert Hooke's 17th century discovery of the cell using a humble compound microscope, light-matter interactions have continuously redefined our understanding of cell biology. Fluorescence microscopy has been particularly transformative and remains an indispensable tool for many cell biologists. The subcellular localization of biomolecules is now routinely visualized simply by manipulating the wavelength of light. Fluorescence polarization microscopy (FPM) extends these capabilities by exploiting another optical property - polarization - allowing researchers to measure not only the location of molecules, but also their organization or alignment within larger cellular structures. With only minor modifications to an existing fluorescence microscope, FPM can reveal the nanoscale architecture, orientational dynamics, conformational changes and interactions of fluorescently labeled molecules in their native cellular environments. Importantly, FPM excels at imaging systems that are challenging to study through traditional structural approaches, such as membranes, membrane proteins, cytoskeletal networks and large macromolecular complexes. In this Review, we discuss key discoveries enabled by FPM, compare and contrast the most common optical setups for FPM, and provide a theoretical and practical framework for researchers to apply this technique to their own research questions.
Keywords: Cellular architecture; Fluorescence polarization; Macromolecular complex; Microscopy; Quantitative fluorescence.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

 , blue) and magnetic (
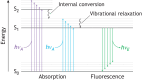
, blue) and magnetic ( , red) fields. (B) Example of Malus' law, showing the relationship between the intensity of unpolarized incident light (I0), the angle (θ) between the transmission axes of two linear polarizers (LP) placed in series, and the final intensity (I) incident on the detector screen. Double-sided blue arrows denote the direction of the electric field. (C) Simplified depiction of anisotropic absorption and emission of light by a fluorophore. The molecule preferentially absorbs photons (hνA) that are polarized parallel to its absorption TDM (
, red) fields. (B) Example of Malus' law, showing the relationship between the intensity of unpolarized incident light (I0), the angle (θ) between the transmission axes of two linear polarizers (LP) placed in series, and the final intensity (I) incident on the detector screen. Double-sided blue arrows denote the direction of the electric field. (C) Simplified depiction of anisotropic absorption and emission of light by a fluorophore. The molecule preferentially absorbs photons (hνA) that are polarized parallel to its absorption TDM ( , blue), transitioning from the ground state (S0) to an excited state (S1), and emits photons (hνE) that are polarized parallel to its emission TDM (
, blue), transitioning from the ground state (S0) to an excited state (S1), and emits photons (hνE) that are polarized parallel to its emission TDM ( , green) when returning to the ground state. Rotation during the fluorescence lifetime causes the output polarization to differ from that of the incident light, as indicated by the double-sided arrows. (D) Classic setup to measure emission anisotropy. An isotropic fluorescent solution is excited with linearly polarized light, leading to selective excitation (photoselection) of molecules oriented similarly to the incident field. The emission intensity is measured at a 90° angle after passing through an analyzer (LP) with its transmission axis oriented parallel (
, green) when returning to the ground state. Rotation during the fluorescence lifetime causes the output polarization to differ from that of the incident light, as indicated by the double-sided arrows. (D) Classic setup to measure emission anisotropy. An isotropic fluorescent solution is excited with linearly polarized light, leading to selective excitation (photoselection) of molecules oriented similarly to the incident field. The emission intensity is measured at a 90° angle after passing through an analyzer (LP) with its transmission axis oriented parallel ( ) or perpendicular (⊥) to the excitation polarization. If the excited molecules remain immobile over their excited-state lifetimes, the emission will be partially polarized in the direction of the incident field. (E) As in D, but for molecules that rotate during their excited-state lifetimes, leading to rotational depolarization of the emitted fluorescence. In D and E, blue double-sided arrows indicate the orientation of the excitation electric field; green double-sided arrows indicate the orientation of the emission electric field after passing through the analyzer.
) or perpendicular (⊥) to the excitation polarization. If the excited molecules remain immobile over their excited-state lifetimes, the emission will be partially polarized in the direction of the incident field. (E) As in D, but for molecules that rotate during their excited-state lifetimes, leading to rotational depolarization of the emitted fluorescence. In D and E, blue double-sided arrows indicate the orientation of the excitation electric field; green double-sided arrows indicate the orientation of the emission electric field after passing through the analyzer.
 ) and emission (
) and emission ( ) TDMs (black double-sided arrows) with azimuthal angles ϕA and ϕE, respectively. The excitation light is propagating along the z-axis. The direction of the excitation electric field (
) TDMs (black double-sided arrows) with azimuthal angles ϕA and ϕE, respectively. The excitation light is propagating along the z-axis. The direction of the excitation electric field ( ) is represented by blue double-sided arrows. The direction of the electric field of the emitted light is shown with green double-sided arrows, labeled corresponding to the intensity (I) components incident on the detector screen. (A) Emission anisotropy imaging. The sample is excited with linearly polarized light and emits light polarized parallel to
) is represented by blue double-sided arrows. The direction of the electric field of the emitted light is shown with green double-sided arrows, labeled corresponding to the intensity (I) components incident on the detector screen. (A) Emission anisotropy imaging. The sample is excited with linearly polarized light and emits light polarized parallel to  . The intensity of the emitted light is measured after passing through an analyzer (LP) oriented parallel (
. The intensity of the emitted light is measured after passing through an analyzer (LP) oriented parallel ( ) or perpendicular (I⊥) to the incident field (
) or perpendicular (I⊥) to the incident field ( ). (B) Fluorescence-detected linear dichroism. Light from an unpolarized source is converted into linearly polarized light by a linear polarizer (LP) with its transmission axis oriented either parallel (
). (B) Fluorescence-detected linear dichroism. Light from an unpolarized source is converted into linearly polarized light by a linear polarizer (LP) with its transmission axis oriented either parallel ( , solid black line) or perpendicular (⊥, dotted black line) to a pre-defined axis (y). The sample is sequentially excited with each excitation polarization (
, solid black line) or perpendicular (⊥, dotted black line) to a pre-defined axis (y). The sample is sequentially excited with each excitation polarization ( and
and  ), and the corresponding emission intensities (
), and the corresponding emission intensities ( and I⊥, respectively) are measured without an analyzer in the emission path. (C) Polarization modulation. Light from a linearly polarized source is passed through a rotatable half-wave plate (HWP; λ/2). The electric field is rotated by two times the angle (ϕEx/2) between it (dotted black line) and the HWP fast axis (solid black line), producing an excitation electric field (
and I⊥, respectively) are measured without an analyzer in the emission path. (C) Polarization modulation. Light from a linearly polarized source is passed through a rotatable half-wave plate (HWP; λ/2). The electric field is rotated by two times the angle (ϕEx/2) between it (dotted black line) and the HWP fast axis (solid black line), producing an excitation electric field ( ) with azimuthal angle ϕEx. The sample is sequentially excited with three or more distinct excitation polarizations (ϕEx) and the corresponding emission intensities (
) with azimuthal angle ϕEx. The sample is sequentially excited with three or more distinct excitation polarizations (ϕEx) and the corresponding emission intensities ( ) are measured without an analyzer in the emission path. (D) Polarization detection. The sample is excited with randomly or circularly polarized light. Light emitted from the sample is passed through an adjustable analyzer (LP) with the transmission axis (solid black line) oriented at angle ϕEm. The sample is sequentially excited using at least three distinct analyzer orientations (ϕEm) and the corresponding emission intensities (
) are measured without an analyzer in the emission path. (D) Polarization detection. The sample is excited with randomly or circularly polarized light. Light emitted from the sample is passed through an adjustable analyzer (LP) with the transmission axis (solid black line) oriented at angle ϕEm. The sample is sequentially excited using at least three distinct analyzer orientations (ϕEm) and the corresponding emission intensities ( ) are measured.
) are measured.References
-
- Abbe, E. (1873). Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Archiv. Für. Mikroskopische Anatomie 9, 413-468. 10.1007/BF02956173 - DOI
-
- Abrahamsson, S., McQuilken, M., Mehta, S. B., Verma, A., Larsch, J., Ilic, R., Heintzmann, R., Bargmann, C. I., Gladfelter, A. S. and Oldenbourg, R. (2015). MultiFocus Polarization Microscope (MF-PolScope) for 3D polarization imaging of up to 25 focal planes simultaneously. Opt. Express 23, 7734-7754. 10.1364/OE.23.007734 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

