Prediction of Ischemic Stroke Functional Outcomes from Acute-Phase Noncontrast CT and Clinical Information
- PMID: 39404632
- PMCID: PMC11535867
- DOI: 10.1148/radiol.240137
Prediction of Ischemic Stroke Functional Outcomes from Acute-Phase Noncontrast CT and Clinical Information
Abstract
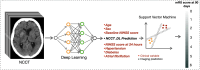
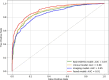
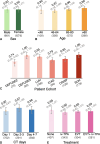
Background Clinical outcome prediction based on acute-phase ischemic stroke data is valuable for planning health care resources, designing clinical trials, and setting patient expectations. Existing methods require individualized features and often involve manually engineered, time-consuming postprocessing activities. Purpose To predict the 90-day modified Rankin Scale (mRS) score with a deep learning (DL) model fusing noncontrast-enhanced CT (NCCT) and clinical information from the acute phase of stroke. Materials and Methods This retrospective study included data from six patient datasets from four multicenter trials and two registries. The DL-based imaging and clinical model was trained by using NCCT data obtained 1-7 days after baseline imaging and clinical data (age; sex; baseline and 24-hour National Institutes of Health Stroke Scale scores; and history of hypertension, diabetes, and atrial fibrillation). This model was compared with models based on either NCCT or clinical information alone. Model-specific mRS score prediction accuracy, mRS score accuracy within 1 point of the actual mRS score, mean absolute error (MAE), and performance in identifying unfavorable outcomes (mRS score, >2) were evaluated. Results A total of 1335 patients (median age, 71 years; IQR, 60-80 years; 674 female patients) were included for model development and testing through sixfold cross validation, with distributions of 979, 133, and 223 patients across training, validation, and test sets in each of the six cross-validation folds, respectively. The fused model achieved an MAE of 0.94 (95% CI: 0.89, 0.98) for predicting the specific mRS score, outperforming the imaging-only (MAE, 1.10; 95% CI: 1.05, 1.16; P < .001) and the clinical information-only (MAE, 1.00; 95% CI: 0.94, 1.05; P = .04) models. The fused model achieved an area under the receiver operating characteristic curve (AUC) of 0.91 (95% CI: 0.89, 0.92) for predicting unfavorable outcomes, outperforming the clinical information-only model (AUC, 0.88; 95% CI: 0.87, 0.90; P < .001) and the imaging-only model (AUC, 0.85; 95% CI: 0.84, 0.87; P < .001). Conclusion A fused DL-based NCCT and clinical model outperformed an imaging-only model and a clinical-information-only model in predicting 90-day mRS scores. © RSNA, 2024 Supplemental material is available for this article. See also the editorial by Lee in this issue.
Conflict of interest statement
Figures
References
-
- Benjamin EJ , Blaha MJ , Chiuve SE , et al . Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association . Circulation 2017. ; 135 ( 10 ): e146 – e603 . [Published corrections appear in Circulation 2017;135(10):e646 and Circulation 2017;136(10):e196.] - PMC - PubMed
-
- Nichols-Larsen DS , Clark PC , Zeringue A , Greenspan A , Blanton S . Factors influencing stroke survivors’ quality of life during subacute recovery . Stroke 2005. ; 36 ( 7 ): 1480 – 1484 . - PubMed
-
- Bernhardt J , Hayward KS , Kwakkel G , et al . Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce . Int J Stroke 2017. ; 12 ( 5 ): 444 – 450 . - PubMed
-
- Langhorne P , Bernhardt J , Kwakkel G . Stroke rehabilitation . Lancet 2011. ; 377 ( 9778 ): 1693 – 1702 . - PubMed
-
- Xie Y , Jiang B , Gong E , et al . JOURNAL CLUB: Use of Gradient Boosting Machine Learning to Predict Patient Outcome in Acute Ischemic Stroke on the Basis of Imaging, Demographic, and Clinical Information . AJR Am J Roentgenol 2019. ; 212 ( 1 ): 44 – 51 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

