Association of incretin-based therapies with hepatobiliary disorders among patients with type 2 diabetes: a case series from the FDA adverse event reporting system
- PMID: 39404734
- PMCID: PMC11623261
- DOI: 10.1530/EC-24-0404
Association of incretin-based therapies with hepatobiliary disorders among patients with type 2 diabetes: a case series from the FDA adverse event reporting system
Abstract
Aim: Incretin therapies, including dipeptidyl peptidase-4 inhibitors (DPP-4is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs), are crucial for type 2 diabetes treatment. Evidence of their association with gallbladder, biliary diseases, and liver injury remains inconsistent. This study evaluated the association between incretin therapies and hepatobiliary adverse events using the FDA's Adverse Event Reporting System (FAERS) data.
Methods: Case reports involving incretin therapies and hepatobiliary events from January 2006 to December 2023 were extracted from FAERS. The association between these agents and hepatobiliary adverse events (hAEs) was analyzed using reporting odds ratios and empirical Bayesian geometric means. Descriptive analyses were conducted to characterize the demographic and clinical features of the hAE cases. Additionally, subgroup analyses calculated reporting odds ratios to evaluate the strength of the association between specific incretin drugs and hAEs.
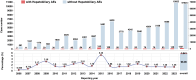
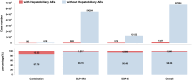
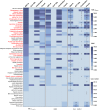
Results: Among 68,351 case reports associated with incretin-based therapies, 1327 (1.941%) involved hepatobiliary adverse events. DPP-4 inhibitors demonstrated statistically significant associations with multiple hepatobiliary events, like cholelithiasis, chronic cholecystitis, and biliary diseases. In contrast, GLP-1 receptor agonists showed weaker associations, primarily linked to gallbladder and biliary disease risks. Subgroup analyses revealed stronger positive correlations with hepatobiliary events for liraglutide and semaglutide among GLP-1 agonists, and for sitagliptin, linagliptin, and vildagliptin among DPP-4 inhibitors. The pooled reporting odds ratio of 2.85 indicated a positive correlation between these drugs and studied adverse events.
Conclusions: This study found statistically significant associations between DPP-4 inhibitors and hepatobiliary adverse events like cholelithiasis and cholecystitis. GLP-1 agonists showed weaker gallbladder/biliary disorder links but higher acute cholecystitis risk. Subgroup analyses revealed varying correlations among specific drugs, potentially dose-dependent. Further large-scale studies are needed to evaluate class effect differences and elucidate mechanisms for guiding clinical use.
Keywords: FDA adverse event reporting system; cholecystitis cholelithiasis; dipeptidyl peptidase-4 inhibitors; glucagon-like peptide-1 receptor agonists; hepatobiliary adverse events; incretin-based therapies; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022. 65 1925–1966. ( 10.1007/s00125-022-05787-2) - DOI - PMC - PubMed
-
- He L Wang J Ping F Yang N Huang J Li Y Xu L Li W & Zhang H. Association of glucagon-like Peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Internal Medicine 2022. 182 513–519. ( 10.1136/10.1001/jamainternmed.2022.0338) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

