Erp57 facilitates ZIKV-induced DNA damage via NS2B/NS3 complex formation
- PMID: 39404735
- PMCID: PMC11520102
- DOI: 10.1080/22221751.2024.2417864
Erp57 facilitates ZIKV-induced DNA damage via NS2B/NS3 complex formation
Abstract
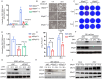
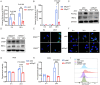
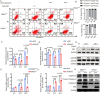
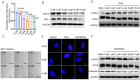
It is believed that DNA double-strand breaks induced by Zika virus (ZIKV) infection in pregnant women is a main reason of brain damage (e.g. microcephaly, severe brain malformation, and neuropathy) in newborn babies [1,2], but its underlying mechanism is poorly understood. In this study, we report that the depletion of ERp57, a member of the protein disulphide isomerase (PDI) family, leads to the limited production of ZIKV in nerve cells. ERp57 knockout not only suppresses viral induced reactive oxygen species (ROS) mediated host DNA damage, but also decreases apoptosis. Strikingly, DNA damage depends on ERp57-bridged complex formation of viral protein NS2B/NS3. LOC14, an ERp57 inhibitor, restricts ZIKV infection and virus-induced DNA damage. Our work reveals an important role of ERp57 in both ZIKV propagation and virus-induced DNA damage, suggesting a potential target against ZIKV infection.
Keywords: DNA damage; ERp57; ROS; ZIKA virus; ZIKV NS2B/NS3 complex; antiviral; apoptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
