The Clinical Genome Resource (ClinGen): Advancing genomic knowledge through global curation
- PMID: 39404758
- PMCID: PMC11984750
- DOI: 10.1016/j.gim.2024.101228
The Clinical Genome Resource (ClinGen): Advancing genomic knowledge through global curation
Abstract
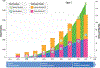
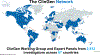
The Clinical Genome Resource (ClinGen) is a National Institutes of Health-funded program founded 10 years ago that defines the clinical relevance of genes and variants for medical and research use. ClinGen working groups develop standards for data sharing and curating genomic knowledge. Expert panels, with >2500 active members from 67 countries, curate the validity of monogenic disease relationships, pathogenicity of genetic variation, dosage sensitivity of genes, and actionability of gene-disease interventions using ClinGen standards, infrastructure, and curation interfaces. Results are available on clinicalgenome.org and classified variants are also submitted to ClinVar, a publicly available database hosted by the National Institutes of Health. As of January 2024, over 2700 genes have been curated (2420 gene-disease relationships for validity, 1557 genes for dosage sensitivity, and 447 gene-condition pairs for actionability), and 5161 unique variants have been classified for pathogenicity. New efforts are underway in somatic cancer, complex disease and pharmacogenomics, and a systematic approach to addressing justice, equity, diversity, and inclusion. ClinGen's knowledge can be used to build evidence-based genetic testing panels, interpret copy-number variation, resolve discrepancies in variant classification, guide disclosure of genomic findings to patients, and assess new predictive algorithms. To get involved in ClinGen activities go to https://www.clinicalgenome.org/start.
Keywords: Actionability; Clinical genetics; Curation; Data sharing; Dosage.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Sharon Plon is a member of the Scientific Advisory Board of Baylor Genetics. Leslie Biesecker receives royalties from Wolters-Kluwer, research support from Merck, and is a member of Illumina Medical Ethics Committee. Adam Buchanan has equity in MeTree and You, Inc. Teri Klein is a member of the Scientific Advisory Board of GalateaBio. Courtney Thaxton receives funds from Pharming, Inc. for work on the Antibody Deficiency Variant Curation Expert Panel. All other ClinGen Consortium authors stated that they have no conflict of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

