Beta cell-specific PAK1 enrichment ameliorates diet-induced glucose intolerance in mice by promoting insulin biogenesis and minimising beta cell apoptosis
- PMID: 39404845
- PMCID: PMC11663170
- DOI: 10.1007/s00125-024-06286-2
Beta cell-specific PAK1 enrichment ameliorates diet-induced glucose intolerance in mice by promoting insulin biogenesis and minimising beta cell apoptosis
Abstract
Aims/hypothesis: p21 (CDC42/RAC1) activated kinase 1 (PAK1) is depleted in type 2 diabetic human islets compared with non-diabetic human islets, and acute PAK1 restoration in the islets can restore insulin secretory function ex vivo. We hypothesised that beta cell-specific PAK1 enrichment in vivo can mitigate high-fat-diet (HFD)-induced glucose intolerance by increasing the functional beta cell mass.
Methods: Human islets expressing exogenous PAK1 specifically in beta cells were used for bulk RNA-seq. Human EndoC-βH1 cells overexpressing myc-tagged PAK1 were used for chromatin immunoprecipitation (ChIP) and ChIP-sequencing (ChIP-seq). Novel doxycycline-inducible beta cell-specific PAK1-expressing (iβPAK1-Tg) mice were fed a 45% HFD pre-induction for 3 weeks and for a further 3 weeks with or without doxycycline induction. These HFD-fed mice were evaluated for GTT, ITT, 6 h fasting plasma insulin and blood glucose, body composition, islet insulin content and apoptosis.
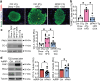
Results: Beta cell-specific PAK1 enrichment in type 2 diabetes human islets resulted in decreased beta cell apoptosis and increased insulin content. RNA-seq showed an upregulation of INS gene transcription by PAK1. Using clonal human beta cells, we found that PAK1 protein was localised in the cytoplasm and the nucleus. ChIP studies revealed that nuclear PAK1 enhanced pancreatic and duodenal homeobox1 (PDX1) and neuronal differentiation 1 (NEUROD1) binding to the INS promoter in a glucose-responsive manner. Importantly, the iβPAK1-Tg mice, when challenged with HFD and doxycycline induction displayed enhanced glucose tolerance, increased islet insulin content and reduced beta cell apoptosis when compared with iβPAK1-Tg mice without doxycycline induction.
Conclusions/interpretation: PAK1 plays an unforeseen and beneficial role in beta cells by promoting insulin biogenesis via enhancing the expression of PDX1, NEUROD1 and INS, along with anti-apoptotic effects, that culminate in increased insulin content and beta cell mass in vivo and ameliorate diet-induced glucose intolerance.
Data availability: The raw and processed RNA-seq data and ChIP-seq data, which has been made publicly available at Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/ , can be accessed in GSE239382.
Keywords: Beta cell; Diet-induced obesity; Human islets; Insulin biogenesis; Insulin gene promoter; NEUROD1; PAK1; PDX1; Type 2 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Acknowledgements: We are grateful to C. V. E. Wright (Department of Cell and Developmental Biology, Vanderbilt University, TN, USA) for providing the PDX1 antibody. We thank the Indiana University School of Medicine Transgenic Mouse core for generating one founder line of TRE-hPAK1 transgenic mice, and the City of Hope Transgenic Mouse Core (Duarte, CA, USA) for generating two additional founder lines. Human islets were provided by the City of Hope Islet Core and by the Integrated Islet Distribution Program (IIDP). Research reported in this publication included work performed in the City of Hope the Electron Microscopy Core, the Integrated Genomics Core, the Comprehensive Metabolic Phenotyping Core, and the Pathology Core supported by the National Cancer Institute of the National Institutes of Health under grant no. P30CA033572. We thank C. S. Jayasena (City of Hope, Duarte, CA, USA) for providing critical feedback and editing the manuscript. Data availability: The raw and processed RNA-seq data and ChIP-seq data, which has been made publicly available at Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/ , can be accessed in GSE239382. Funding: Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This work was supported by the National Institutes of Health grants to DCT (DK067912, DK112917 and DK102233) and SD (R01DK120523). The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report. Authors’ relationships and activities: The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: The study was conceptualised by DCT and MA. The funding was acquired by DCT. Experiments were performed by EMM, MA, PAG and SD. Bioinformatics analysis was performed by SB. Resources were provided by RS. The project was supervised by DCT. The original draft of this manuscript was written by DCT and MA. DCT, MA, EMM, PAG, RS, SB and SD were involved in the data analysis, discussion and editing of the manuscript. All authors approved the final version of the manuscript. DCT is the guarantor of this work and takes full responsibility for the manuscript.
Figures





References
-
- International Diabetes Federation (2021) IDF Diabetes Atlas, 10th edition. available from www.diabetesatlas.org. Accessed 24, April. 2023
-
- CDC (2022) Centres for Disease Control and Prevention. National Diabetes Statistics Report website. https://www.cdc.gov/diabetes/satistics-report/index.html. Accessed 12 December. 2022
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

