Oxidative stress initiates hemodynamic change in CKD-induced heart disease
- PMID: 39404904
- PMCID: PMC11628585
- DOI: 10.1007/s00395-024-01085-7
Oxidative stress initiates hemodynamic change in CKD-induced heart disease
Abstract
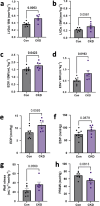
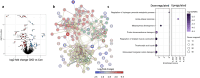
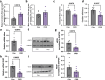
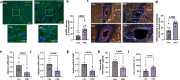
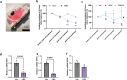
Chronic kidney disease (CKD) predisposes to cardiac remodeling and coronary microvascular dysfunction. Studies in swine identified changes in microvascular structure and function, as well as changes in mitochondrial structure and oxidative stress. However, CKD was combined with metabolic derangement, thereby obscuring the contribution of CKD alone. Therefore, we studied the impact of CKD on the heart and combined proteome studies with measurement of cardiac function and perfusion to identify processes involved in cardiac remodeling in CKD. CKD was induced in swine at 10-12 weeks of age while sham-operated swine served as controls. 5-6 months later, left ventricular (LV) function and coronary flow reserve were measured. LC-MS-MS-based proteomic analysis of LV tissue was performed. LV myocardium and kidneys were histologically examined for interstitial fibrosis and oxidative stress. Renal embolization resulted in mild chronic kidney injury (increased fibrosis and urinary NGAL). PV loops showed LV dilation and increased wall stress, while preload recruitable stroke work was impaired in CKD. Quantitative proteomic analysis of LV myocardium and STRING pre-ranked functional analysis showed enrichments in pathways related to contractile function, reactive oxygen species, and extracellular matrix (ECM) remodeling, which were confirmed histologically and associated with impaired total anti-oxidant capacity. H2O2 exposure of myocardial slices from CKD, but not normal swine, impaired contractile function. Furthermore, in CKD, mitochondrial proteins were downregulated suggesting mitochondrial dysfunction which was associated with higher basal coronary blood flow. Thus, mild CKD induces alterations in mitochondrial proteins along with contractile proteins, oxidative stress and ECM remodeling, that were associated with changes in cardiac function and perfusion.
Keywords: Cardiac remodeling; Chronic kidney disease; Coronary flow reserve; Oxidative stress; Proteomics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Canton M, Neverova I, Menabò R, Van Eyk J, Di Lisa F (2004) Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286:H870–H877. 10.1152/ajpheart.00714.2003 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

