How is Excitotoxicity Being Modelled in iPSC-Derived Neurons?
- PMID: 39405005
- PMCID: PMC11480214
- DOI: 10.1007/s12640-024-00721-3
How is Excitotoxicity Being Modelled in iPSC-Derived Neurons?
Abstract
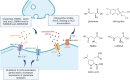
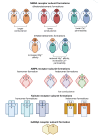
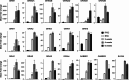
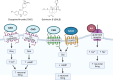
Excitotoxicity linked either to environmental causes (pesticide and cyanotoxin exposure), excitatory neurotransmitter imbalance, or to intrinsic neuronal hyperexcitability, is a pathological mechanism central to neurodegeneration in amyotrophic lateral sclerosis (ALS). Investigation of excitotoxic mechanisms using in vitro and in vivo animal models has been central to understanding ALS mechanisms of disease. In particular, advances in induced pluripotent stem cell (iPSC) technologies now provide human cell-based models that are readily amenable to environmental and network-based excitotoxic manipulations. The cell-type specific differentiation of iPSC, combined with approaches to modelling excitotoxicity that include editing of disease-associated gene variants, chemogenetics, and environmental risk-associated exposures make iPSC primed to examine gene-environment interactions and disease-associated excitotoxic mechanisms. Critical to this is knowledge of which neurotransmitter receptor subunits are expressed by iPSC-derived neuronal cultures being studied, how their activity responds to antagonists and agonists of these receptors, and how to interpret data derived from multi-parameter electrophysiological recordings. This review explores how iPSC-based studies have contributed to our understanding of ALS-linked excitotoxicity and highlights novel approaches to inducing excitotoxicity in iPSC-derived neurons to further our understanding of its pathological pathways.
Keywords: AMPAR; Calcium; DREADDs; Glutamate; Kainic acid; NMDAR.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neural Differentiation and spinal cord organoid generation from induced pluripotent stem cells (iPSCs) for ALS modelling and inflammatory screening.Mol Neurobiol. 2024 Jul;61(7):4732-4749. doi: 10.1007/s12035-023-03836-4. Epub 2023 Dec 21. Mol Neurobiol. 2024. PMID: 38127186
-
Modelling and treating amyotrophic lateral sclerosis through induced-pluripotent stem cells technology.Curr Stem Cell Res Ther. 2016;11(4):301-12. doi: 10.2174/1574888x10666150528144303. Curr Stem Cell Res Ther. 2016. PMID: 26018231 Review.
-
A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells.Mol Cell Neurosci. 2013 Sep;56:355-64. doi: 10.1016/j.mcn.2013.07.007. Epub 2013 Jul 25. Mol Cell Neurosci. 2013. PMID: 23891805 Free PMC article.
-
Recent insights from human induced pluripotent stem cell models into the role of microglia in amyotrophic lateral sclerosis.Bioessays. 2024 Jul;46(7):e2400054. doi: 10.1002/bies.202400054. Epub 2024 May 7. Bioessays. 2024. PMID: 38713169 Review.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
Cited by
-
An injectable 2.5% cross-linked polyacrylamide hydrogel (2.5 iPAAG) demonstrates no neurotoxicity in human induced pluripotent stem cells-derived iCell® GlutaNeurons.Front Toxicol. 2025 Jun 23;7:1585430. doi: 10.3389/ftox.2025.1585430. eCollection 2025. Front Toxicol. 2025. PMID: 40626196 Free PMC article.
References
-
- Achilli F, Boyle S, Kieran D, Chia R, Hafezparast M, Martin JE, Schiavo G, Greensmith L, Bickmore W, Fisher EM (2005) The SOD1 transgene in the G93A mouse model of amyotrophic lateral sclerosis lies on distal mouse chromosome 12. Amyotroph Lateral Scler 6:111–114. 10.1080/14660820510035351 - PubMed
-
- Al-Chalabi A, Lewis CM (2011) Modelling the effects of penetrance and family size on rates of sporadic and familial disease. Human Hered 71:281–288. 10.1159/000330167 - PubMed
-
- Amalyan S, Tamboli S, Lazarevich I, Topolnik D, Bouman LH, Topolnik L (2022) Enhanced motor cortex output and disinhibition in asymptomatic female mice with C9orf72 genetic expansion. Cell Rep 40. 10.1016/j.celrep.2022.111043 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

