The win ratio in cardiology trials: lessons learnt, new developments, and wise future use
- PMID: 39405050
- PMCID: PMC11578645
- DOI: 10.1093/eurheartj/ehae647
The win ratio in cardiology trials: lessons learnt, new developments, and wise future use
Abstract
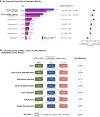
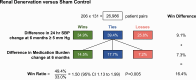
The win ratio method for analysing a composite clinical hierarchy of outcomes is growing in popularity especially in cardiovascular trials. This article gives a perspective on its use so far and the issues derived from that experience. Specifically, it focuses on the limitations of a conventional composite outcome; how does the win ratio work, what does it mean, and how to display its findings; guidance on choosing an appropriate clinical hierarchy of outcomes including clinical events, quantitative outcomes, and other options; the additional value of the win difference as a measure of absolute benefit: extension to stratified win ratio, subgroup analysis, matched win ratio, and covariate adjustment; determining trial size for a win ratio outcome; specific insights such as adaptive designs, use of repeat events, and use of margins and time averages for quantitative outcomes; a critique of potential misuses; availability of statistical software; and a statistical appendix on the methodological details. Throughout, each principle is illustrated by examples from specific cardiology trials. The article concludes with a set of recommendations for future use of the win ratio.
Keywords: Clinical trial; Hierarchical composite outcome; Presentation and interpretation; Statistical methods; Systematic review; Win ratio.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

