Selective colonization of microplastics, wood and glass by antimicrobial-resistant and pathogenic bacteria
- PMID: 39405105
- PMCID: PMC11477370
- DOI: 10.1099/mic.0.001506
Selective colonization of microplastics, wood and glass by antimicrobial-resistant and pathogenic bacteria
Abstract
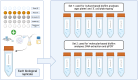
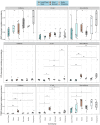
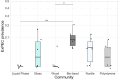
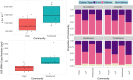
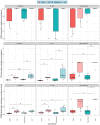
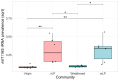
The Plastisphere is a novel niche whereby microbial communities attach to plastic debris, including microplastics. These communities can be distinct from those found in the surrounding environment or those attached to natural substrates and may serve as a reservoir of both pathogenic and antimicrobial-resistant (AMR) bacteria. Owing to the frequent omission of appropriate comparator particles (e.g. natural substrates) in previous studies, there is a lack of empirical evidence supporting the unique risks posed by microplastics in terms of enrichment and spread of AMR pathogens. This study investigated selective colonization by a sewage community on environmentally sampled microplastics with three different polymers, sources and morphologies, alongside natural substrate (wood), inert substrate (glass) and free-living/planktonic community controls. Culture and molecular methods (quantitative polymerase chain reaction (qPCR)) were used to ascertain phenotypic and genotypic AMR prevalence, respectively, and multiplex colony PCR was used to identify extra-intestinal pathogenic Escherichia coli (ExPECs). From this, polystyrene and wood particles were found to significantly enrich AMR bacteria, whereas sewage-sourced bio-beads significantly enriched ExPECs. Polystyrene and wood were the least smooth particles, and so the importance of particle roughness on AMR prevalence was then directly investigated by comparing the colonization of virgin vs artificially weathered polyethylene particles. Surface weathering did not have a significant effect on the AMR prevalence of colonized particles. Our results suggest that the colonization of plastic and non-plastic particles by AMR and pathogenic bacteria may be enhanced by substrate-specific traits.
Keywords: Escherichia coli; Plastisphere; antimicrobial resistance; biofilm; extra-intestinal pathogen; microplastic; wastewater.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Microplastic biofilm, associated pathogen and antimicrobial resistance dynamics through a wastewater treatment process incorporating a constructed wetland.Water Res. 2023 May 15;235:119936. doi: 10.1016/j.watres.2023.119936. Epub 2023 Apr 3. Water Res. 2023. PMID: 37028211
-
Selection for antimicrobial resistance in the plastisphere.Sci Total Environ. 2024 Jan 15;908:168234. doi: 10.1016/j.scitotenv.2023.168234. Epub 2023 Nov 2. Sci Total Environ. 2024. PMID: 37924893 Review.
-
Effects of microplastic concentration, composition, and size on Escherichia coli biofilm-associated antimicrobial resistance.Appl Environ Microbiol. 2025 Apr 23;91(4):e0228224. doi: 10.1128/aem.02282-24. Epub 2025 Mar 11. Appl Environ Microbiol. 2025. PMID: 40067049 Free PMC article.
-
Taxonomic variation, plastic degradation, and antibiotic resistance traits of plastisphere communities in the maturation pond of a wastewater treatment plant.Appl Environ Microbiol. 2024 Oct 23;90(10):e0071524. doi: 10.1128/aem.00715-24. Epub 2024 Sep 27. Appl Environ Microbiol. 2024. PMID: 39329490 Free PMC article.
-
Microplastic-associated pathogens and antimicrobial resistance in environment.Chemosphere. 2022 Mar;291(Pt 2):133005. doi: 10.1016/j.chemosphere.2021.133005. Epub 2021 Nov 20. Chemosphere. 2022. PMID: 34813845 Review.
References
-
- Deng H, Fu Q, Zhang Y, Li D, He J, et al. Bacterial communities on polyethylene microplastics in mangrove ecosystems as a function of exposure sites: compositions and ecological functions. J Environ Chem Eng. 2022;10:107924. doi: 10.1016/j.jece.2022.107924. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

