Type 2 innate immunity promotes the development of pulmonary fibrosis in Hermansky-Pudlak syndrome
- PMID: 39405112
- PMCID: PMC11601950
- DOI: 10.1172/jci.insight.178381
Type 2 innate immunity promotes the development of pulmonary fibrosis in Hermansky-Pudlak syndrome
Abstract
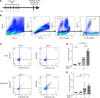
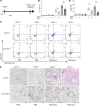
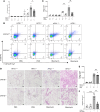
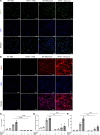
Hermansky-Pudlak syndrome (HPS), particularly types 1 and 4, is characterized by progressive pulmonary fibrosis, a major cause of morbidity and mortality. However, the precise mechanisms driving pulmonary fibrosis in HPS are not fully elucidated. Our previous studies suggested that CHI3L1-driven fibroproliferation may be a notable factor in HPS-associated fibrosis. This study aimed to explore the role of CHI3L1-CRTH2 interaction on type 2 innate lymphoid cells (ILC2s) and explored the potential contribution of ILC2-fibroblast crosstalk in the development of pulmonary fibrosis in HPS. We identified ILC2s in lung tissues from patients with idiopathic pulmonary fibrosis and HPS. Using bleomycin-challenged WT and Hps1-/- mice, we observed that ILC2s were recruited and appeared to contribute to fibrosis development in the Hps1-/- mice, with CRTH2 playing a notable role in ILC2 accumulation. We sorted ILC2s, profiled fibrosis-related genes and mediators, and conducted coculture experiments with primary lung ILC2s and fibroblasts. Our findings suggest that ILC2s may directly stimulate the proliferation and differentiation of primary lung fibroblasts partially through amphiregulin-EGFR-dependent mechanisms. Additionally, specific overexpression of CHI3L1 in the ILC2 population using the IL-7Rcre driver, which was associated with increased fibroproliferation, indicates that ILC2-mediated, CRTH2-dependent mechanisms might contribute to optimal CHI3L1-induced fibroproliferative repair in HPS-associated pulmonary fibrosis.
Keywords: Fibrosis; Genetic diseases; Immunology; Innate immunity; Pulmonology.
Conflict of interest statement
Figures




References
-
- Gahl W, Huizing M. Hermansky-Pudlak syndrome. In: Adam MP, et al, eds. GeneReviews. University of Washington; 2012.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

