EMC3 regulates trafficking and pulmonary toxicity of the SFTPCI73T mutation associated with interstitial lung disease
- PMID: 39405113
- PMCID: PMC11601914
- DOI: 10.1172/JCI173861
EMC3 regulates trafficking and pulmonary toxicity of the SFTPCI73T mutation associated with interstitial lung disease
Abstract
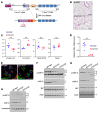
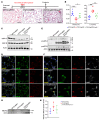
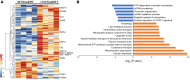
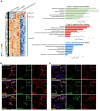
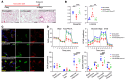
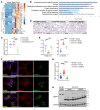
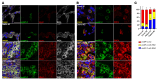
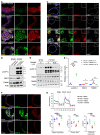
The most common mutation in surfactant protein C gene (SFTPC), SFTPCI73T, causes interstitial lung disease with few therapeutic options. We previously demonstrated that EMC3, an important component of the multiprotein endoplasmic reticulum membrane complex (EMC), is required for surfactant homeostasis in alveolar type 2 epithelial (AT2) cells at birth. In the present study, we investigated the role of EMC3 in the control of SFTPCI73T metabolism and its associated alveolar dysfunction. Using a knock-in mouse model phenocopying the I73T mutation, we demonstrated that conditional deletion of Emc3 in AT2 cells rescued alveolar remodeling/simplification defects in neonatal and adult mice. Proteomic analysis revealed that Emc3 depletion reversed the disruption of vesicle trafficking pathways and rescued the mitochondrial dysfunction associated with I73T mutation. Affinity purification-mass spectrometry analysis identified potential EMC3 interacting proteins in lung AT2 cells, including valosin containing protein (VCP) and its interactors. Treatment of SftpcI73T knock-in mice and SFTPCI73T-expressing iAT2 cells derived from SFTPCI73T patient-specific iPSCs with the VCP inhibitor CB5083 restored alveolar structure and SFTPCI73T trafficking, respectively. Taken together, the present work identifies the EMC complex and VCP in the metabolism of the disease-associated SFTPCI73T mutant, providing therapeutical targets for SFTPCI73T-associated interstitial lung disease.
Keywords: Cell biology; Protein traffic; Pulmonology.
Conflict of interest statement
Figures
References
-
- Daniels CB, Orgeig S. Pulmonary surfactant: the key to the evolution of air breathing. News Physiol Sci. 2003;18:151–157. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

