TREM2 aggravates sepsis by inhibiting fatty acid oxidation via the SHP1/BTK axis
- PMID: 39405126
- PMCID: PMC11684808
- DOI: 10.1172/JCI159400
TREM2 aggravates sepsis by inhibiting fatty acid oxidation via the SHP1/BTK axis
Abstract
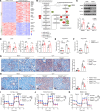
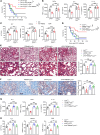
Impaired fatty acid oxidation (FAO) and the therapeutic benefits of FAO restoration have been revealed in sepsis. However, the regulatory factors contributing to FAO dysfunction during sepsis remain inadequately clarified. In this study, we identified a subset of lipid-associated macrophages characterized by high expression of trigger receptor expressed on myeloid cells 2 (TREM2) and demonstrated that TREM2 acted as a suppressor of FAO to increase the susceptibility to sepsis. TREM2 expression was markedly upregulated in sepsis patients and correlated with the severity of sepsis. Knockout of TREM2 in macrophages improved the survival rate and reduced inflammation and organ injuries of sepsis mice. Notably, TREM2-deficient mice exhibited decreased triglyceride accumulation and an enhanced FAO rate. Further observations showed that the blockade of FAO substantially abolished the alleviated symptoms observed in TREM2-knockout mice. Mechanically, we demonstrated that TREM2 interacted with the phosphatase SHP1 to inhibit bruton tyrosine kinase-mediated (BTK-mediated) FAO in sepsis. Our findings expand the understanding of FAO dysfunction in sepsis and reveal TREM2 as a critical regulator of FAO that may provide a promising target for the clinical treatment of sepsis.
Keywords: Fatty acid oxidation; Infectious disease.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

